Abstract
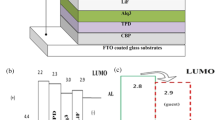
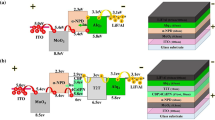
The UV-Vis absorption and photoluminescence (PL) spectra of 2,3-bis(9,9-dihexyl-9H-fluoren-2-yl) quinoxaline (Py), 2,3-bis(9,9-dihexyl-9H-fluoren-2-yl)-6,7-difluoroquinoxaline (F2Py) and 2,3-bis(9,9-dihexyl-9H-fluoren-2-yl)-5,6,7,8-tetrafluoroquinoxaline (F4Py), which are fluorene molecular derivatives with conjugated structure, were investigated. For further investigation of the influences of fluorine auxochrome in fluorene molecular electroluminescent material on optoelectronic property, the electroluminescence (EL) characteristics of materials were studied by double-layer organic light-emitting diodes (OLEDs) using N,N′-Di-[(1-naphthalenyl)-N,N′-diphenyl]-(1,1′-biphenyl)-4,4′-diamine (NPB) as a hole transporting layer (HTL) with the conventional vacuum deposition method. The results showed that the absorption and PL spectra of materials in film state red shifted with fluorine substituents increased in molecule configuration. The performance of OLEDs is as follows: at a bias voltage of 5 V, Py emitted a blue-green light at 508 nm with the Commission Internationale d’Eclairage (CIE) coordinates of (0.23, 0.43) and full width at half maximum (FWHM) of 100 nm. The device had a turn-on voltage (defined as the drive voltage at the luminance of 1 cd/m2) of 4.8 V, a luminance of 129 cd/m2 with a current density of 59 mA/cm2 at 10 V, and a maximum luminous efficiency of 0.18 lm/W at 5.4 V. F2Py and F4Py emitted a green light peaking at 544 nm and a yellow light at 570 nm at 5 V, with the CIE coordinates of (0.38, 0.56) and (0.44, 0.49), and FWHM of 103 and 117 nm, respectively. The F2Py and F4Py devices had a turn-on voltage of 4 and 2 V, a luminance of 557 and 3300 cd/m2 with a current density of 100 and 880 mA/cm2 at 10 V, and a maximum luminous efficiency of 0.22 lm/W at 7.6 V and 0.53 lm/W at 2 V, respectively.
Similar content being viewed by others
References
Tang C W, VanSlyke S A. Organic electroluminescent diodes. Appl Phys Lett, 1987, 51(12): 913–915
Wang J, Jiang Y D, Yu J S, et al. Low operating voltage bright organic light-emitting diode using a novel iridium complex doped in 4,4′-bis[N-1-napthyl-N-phenyl-amino]biphenyl. Appl Phys Lett, 2007, 91: 131105
Yu J, Li W, Jiang Y, et al. Bright-yellow organic light-emitting device using novel silole derivative as emitter. Jpn J Appl Phys, 2007, 46(2): L31–L33
Shirota Y, Kuwabara Y, India H. Multilayered organic electroluminescent device using a novel starburst molecule, 4,4′,4′-tris(3-methylphenylphenylamino)triphenylamine, as a hole transport material. Appl Phys Lett, 1994, 65: 807–809
Hashimoto Y, Hamagaki M, Sakakibara T. Effect of ionization potential of hole transport layer on device characteristics of organic light emitting diode with oxygen plasma treated indium tin oxide. Jpn J Appl Phys, 2001, 40(7): 4720–4725
Lou S, Yu J, Li W, et al. Study of organic light-emitting diodes based on new hole transport layer (in Chinese). Acta Opt Sin, 2007, 27(8): 1455–1458
Chen H. The effects of fluorination on the electron transport properties of perylene diimides (in Chinese). Proceedings of the Academic Dissertation and Bulletin of Polymer of China, 2005
Fang Q, Xu B, Jiang B, et al. A novel fluorene derivative containing four triphenylamine groups: Highly thermostable blue emitter with hole-transporting ability for organic light-emitting diode (OLED). Synth Met, 2005, 155: 206–210
Han M, Lee S, Jung J, et al. Effect of a perfluorocyclopentene core unit on the structures and photoluminescence of fluorene-and anthracene-based compounds. Tetrahedron, 2006, 62: 9769–9777
Tang C, Liu F, **a Y, et al. Fluorene-substituted pyrenes-Novel pyrene derivatives as emitters in nondoped blue OLEDs. Org Electron, 2006, 7: 155–162
Gong X, Iyer P K, Moses D, et al. Stabilized blue emission from polyfluorene-based light-emitting diodes: Elimination of fluorenone defects. Adv Funct Mater, 2003, 13: 325–330
Kim J S, Cacialli F, Cola A, et al. Hall measurements of treated indium tin oxide surfaces. Synth Met, 2000, 111–112: 363–367
Nguyen T Pr, Rendu P Le. Thermal and chemical treatment of ITO substrates for improvement of OLED performance. Synth Met, 2003, 138: 229–232
Leclère Ph, Surin M, Brocorens P, et al. Supramolecular assembly of conjugated polymers: From molecular engineering to solid-state properties. Mat Sci Eng R, 2006, 55: 1–56
Weinfurtner K, Fujikawa H, Tokito S, et al. Highly efficient pure blue electroluminescence from polyfluorene: Influence of the molecular weight distribution on the aggregation tendency. Appl Phys Lett, 2000, 76(18): 2502–2504
Ahn K H, Ryu G Y, Youn S W, et al. The conjugation effects on the luminescence properties of oligophenylenes for the OLED. Mat Sci Eng C, 2004, 24: 163–165
Jiang H, Feng J, Wei W, et al. The synthesis of a promising electron transporting-hole blocking material-2,7-bis(9,9-didodecyl-2-(2,4-difluorophenyl)-fluoren)-fluoren (in Chinese). Proceedings of the Academic Dissertation and Bulletin of Polymer of China, 2005
Acharya S, Bhattacharjee D, Talapatra G B. Spectroscopic study of non-amphiphilic 9-phenyl fluorene assembled in Langmuir-Blodgett films in two different matrices: Dimer and excimer formation. Chem Phys Lett, 2003, 372(1–2): 97–103
Yip W T, Levy D H. Excimer/exciplex formation in van der Waals dimers of aromatic molecules. J Phys Chem, 1996, 100(28): 11539–11545
Fowler R H, Nordheim L W. Electron emission in intense electric fields. Proc Royal Soc London Ser A, 1928, 119: 173–181
Shi M, Chen H, Wang M. Influences of different fluorinated substituents on electron mobility of perylene diimides (in Chinese). Acta Chim Sin, 2006, 64(8): 721–726
Xu M, Li W, Li M, et al. A new iridium complex with trifluoromethylsubstituted 2-benzo[b]thiophen-2-yl-pyridine ligand and its application in OLEDs (in Chinese). Chin J Lumin, 2007, 28(3): 433–436
Pope M, Swenberg C E. Electronic Processes in Organic Crystals. Oxford: Clarendon Press, 1982
Huang C, Li F, Huang W. Introduction to Organic Light-Emitting Materials and Devices (in Chinese). Shanghai: Fudan University Press, 2005
Yang J, Shen J. Effects of the hole barrier in bilayer organic lightemitting devices. J Phys D Appl Phys, 2000, 33: 1768–1772
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 60425101, 20674049), Program for New Century Excellent Talents in University (Grant No. NCET-06-0812) and Young Excellence Project of UESTC (Grant No. 060206)
About this article
Cite this article
Lou, S., Yu, J., Jiang, Y. et al. Influences of fluorine auxochrome in fluorene molecular electroluminescent material on optoelectronic property. Chin. Sci. Bull. 53, 2940–2945 (2008). https://doi.org/10.1007/s11434-008-0395-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-008-0395-1