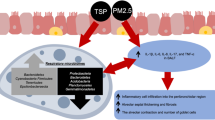
Abstract
Particulate matters (PMs) are significant components of air pollution in the urban environment. PMs with aerodynamic diameter less than 2.5 μm (PM2.5) can penetrate to the alveolar area and introduce numerous compounds to the pneumocystis that can initiate inflammatory response. There are several questions about this exposure as follows: does PM2.5-induced inflammation lead to a specific disease? If yes, what is the form of the progressed disease? This systematic review was designed and conducted to respond to these questions. Four databases, including Web of Science, Scopus, PubMed, and Embase, were reviewed systematically to find the related articles. According to the included articles, the only available data on the inflammatory effects of PM2.5 comes from either in vitro or animal studies. Both types of studies have shown that the induced inflammation is type I and includes secretion of proinflammatory cytokines. The exposure duration of longer than 28 weeks was not observed in any of the reviewed studies. However, as there is not a specific antigenic component in the urban particulate matters and based on the available evidence, the antigen-presenting is not a common process in the inflammatory responses to PM2.5. Therefore, neither signaling to repair cells such as fibroblasts nor over-secretion of extracellular matrix (ECM) proteins can occur following PM2.5-induced inflammation. These pieces of evidence weaken the probability of the development of fibrotic diseases. On the other hand, permanent inflammation induces the destruction of ECM and alveolar walls by over-secretion of protease enzymes and therefore results in progressive obstructive effects.



Similar content being viewed by others
Data availability
All of the used datasets during the current study are available online.
References
Abbas AK, Lichtman AH, Pillai S (2019a) Basic immunology e-book: functions and disorders of the immune system. Elsevier Health Sciences
Abbas I, Badran G, Verdin A, Ledoux F, Roumie M, Lo Guidice JM, Courcot D, Garçon G (2019b) In vitro evaluation of organic extractable matter from ambient PM2. 5 using human bronchial epithelial BEAS-2B cells: cytotoxicity, oxidative stress, pro-inflammatory response, genotoxicity, and cell cycle deregulation. Environ Res 171:510–522
Akhtar US, McWhinney RD, Rastogi N, Abbatt JP, Evans GJ, Scott JA (2010) Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal Toxicol 22:37–47
Chilosi M, Poletti V, Rossi A (2012) The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res 13:3
Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA III, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH (2017) Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389:1907–1918
Cosio MG, Majo J, Cosio MG (2002) Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest 121:160S–165S
Cottin V, Hansell DM, Sverzellati N, Weycker D, Antoniou KM, Atwood M, Oster G, Kirchgaessler KU, Collard HR, Wells AU (2017) Effect of emphysema extent on serial lung function in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196:1162–1171
Dagher Z, Garçon G, Billet S, Gosset P, Ledoux F, Courcot D, Aboukais A, Shirali P (2006) Activation of different pathways of apoptosis by air pollution particulate matter (PM2. 5) in human epithelial lung cells (L132) in culture. Toxicology 225:12–24
Dagher Z, Garçon G, Gosset P, Ledoux F, Surpateanu G, Courcot D, Aboukais A, Puskaric E, Shirali P (2005) Pro-inflammatory effects of Dunkerque city air pollution particulate matter 2.5 in human epithelial lung cells (L132) in culture. J Appl Toxicol 25:166–175
Deiuliis JA et al (2012) Pulmonary T cell activation in response to chronic particulate air pollution. Am J Phys Lung Cell Mol Phys 302:L399–L409
Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, Chen D, Ding W (2013) PM2. 5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol in Vitro 27:1762–1770
Dergham M, Lepers C, Verdin A, Cazier F, Billet S, Courcot D, Shirali P, Garçon G (2015) Temporal–spatial variations of the physicochemical characteristics of air pollution particulate matter (PM2. 5–0.3) and toxicological effects in human bronchial epithelial cells (BEAS-2B). Environ Res 137:256–267
Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM (2006) Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama 295:1127–1134
El-Bayoumi E, Silvestri GA (2008) Bronchoscopy for the diagnosis and staging of lung cancer. In: Seminars in respiratory and critical care medicine. vol 03. © Thieme Medical Publishers, pp 261-270
Feng S, Gao D, Liao F, Zhou F, Wang X (2016) The health effects of ambient PM2. 5 and potential mechanisms. Ecotoxicol Environ Saf 128:67–74
Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD (2016) Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 15:35–50
Gao J, Peng X, Chen G, Xu J, Shi G-L, Zhang Y-C, Feng Y-C (2016) Insights into the chemical characterization and sources of PM2. 5 in Bei**g at a 1-h time resolution. Sci Total Environ 542:162–171
Gavett SH, Haykal-Coates N, Copeland LB, Heinrich J, Gilmour MI (2003) Metal composition of ambient PM2. 5 influences severity of allergic airways disease in mice. Environ Health Perspect 111:1471–1477
Geng H, Meng Z, Zhang Q (2006) In vitro responses of rat alveolar macrophages to particle suspensions and water-soluble components of dust storm PM2. 5. Toxicol in Vitro 20:575–584
Gualtieri M, Øvrevik J, Holme JA, Perrone MG, Bolzacchini E, Schwarze PE, Camatini M (2010) Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol in Vitro 24:29–39
Happo M et al (2010) Seasonal variation in chemical composition of size-segregated urban air particles and the inflammatory activity in the mouse lung. Inhal Toxicol 22:17–32
He M, Ichinose T, Kobayashi M, Arashidani K, Yoshida S, Nishikawa M, Takano H, Sun G, Shibamoto T (2016) Differences in allergic inflammatory responses between urban PM2. 5 and fine particle derived from desert-dust in murine lungs. Toxicol Appl Pharmacol 297:41–55
He M, Ichinose T, Yoshida S, Ito T, He C, Yoshida Y, Arashidani K, Takano H, Sun G, Shibamoto T (2017) PM2. 5-induced lung inflammation in mice: differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol 37:1203–1218
He R-W, Shirmohammadi F, Gerlofs-Nijland ME, Sioutas C, Cassee FR (2018) Pro-inflammatory responses to PM0. 25 from airport and urban traffic emissions. Sci Total Environ 640:997–1003
Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, Deng C (2016) Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med 239:117–125
Hou T, Liao J, Zhang C, Sun C, Li X, Wang G (2018) Elevated expression of miR-146, miR-139 and miR-340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. Int Immunopharmacol 54:68–77
Hsu C-Y et al (2017) Ambient PM2. 5 in the residential area near industrial complexes: spatiotemporal variation, source apportionment, and health impact. Sci Total Environ 590:204–214
Huang K-L, Liu S-Y, Chou CC, Lee Y-H, Cheng T-J (2017a) The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS One 12
Huang Q, Chi Y, Deng J, Liu Y, Lu Y, Chen J, Dong S (2017b) Fine particulate matter 2.5 exerted its toxicological effect by regulating a new layer, long non-coding RNA. Sci Rep 7:1–9
** W, Wang L (2019) Role of rapid on site evaluation in preliminary diagnosis of pulmonary infection during bronchoscopy. Eur Respiratory Soc
Kim I, Lee K, Lee S, Kim SD (2019) Characteristics and health effects of PM2. 5 emissions from various sources in Gwangju, South Korea. Sci Total Environ 696:133890
Kim KM, Pae H-O, Zheng M, Park R, Kim Y-M, Chung H-T (2007) Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R–like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res 101:919–927
King TE Jr, Pardo A, Selman M (2011) Idiopathic pulmonary fibrosis. Lancet 378:1949–1961
Kulkarni N, Pierse N, Rushton L, Grigg J (2006) Carbon in airway macrophages and lung function in children. N Engl J Med 355:21–30
Kumar V, Abbas AK, Fausto N, Aster JC (2014) Robbins and Cotran pathologic basis of disease, professional edition e-book. elsevier health sciences
Lauer FT, Mitchell LA, Bedrick E, McDonald JD, Lee WY, Li WW, Olvera H, Amaya MA, Berwick M, Gonzales M, Currey R, **itore NE Jr, Burchiel SW (2009) Temporal–spatial analysis of US–Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol Appl Pharmacol 238:1–10
Leclercq B, Alleman LY, Perdrix E, Riffault V, Happillon M, Strecker A, Lo-Guidice JM, Garçon G, Coddeville P (2017) Particulate metal bioaccessibility in physiological fluids and cell culture media: toxicological perspectives. Environ Res 156:148–157
Lewis JB, Bodine JS, Gassman JR, Muñoz SA, Milner DC, Dunaway TM, Egbert KM, Monson TD, Broberg DS, Arroyo JA, Reynolds PR (2018) Transgenic up-regulation of Claudin-6 decreases fine diesel particulate matter (DPM)-induced pulmonary inflammation. Environ Sci Pollut Res 25:18179–18188
Li D, Zhang R, Cui L, Chu C, Zhang H, Sun H, Luo J, Zhou L, Chen L, Cui J, Chen S, Mai B, Chen S, Yu J, Cai Z, Zhang J, Jiang Y, Aschner M, Chen R, Zheng Y, Chen W (2019) Multiple organ injury in male C57BL/6J mice exposed to ambient particulate matter in a real-ambient PM exposure system in Shijiazhuang, China. Environ Pollut 248:874–887
Li H, Wang Q', Yang M, Li F, Wang J, Sun Y, Wang C, Wu H, Qian X (2016) Chemical characterization and source apportionment of PM2. 5 aerosols in a megacity of Southeast China. Atmos Res 181:288–299
Lin G, Fu J, Jiang D, Hu W, Dong D, Huang Y, Zhao M (2014) Spatio-temporal variation of PM2. 5 concentrations and their relationship with geographic and socioeconomic factors in China. Int J Environ Res Public Health 11:173–186
Liu Q, Baumgartner J, Zhang Y, Schauer JJ (2016) Source apportionment of Bei**g air pollution during a severe winter haze event and associated pro-inflammatory responses in lung epithelial cells. Atmos Environ 126:28–35
Liu S, Zhang W, Zhang F, Roepstorff P, Yang F, Lu Z, Ding W (2019) TMT-based quantitative proteomics analysis reveals airborne PM2. 5-induced pulmonary fibrosis. International journal of environmental research and public health 16:98
McMillan SJ et al (2004) Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol 172:2586–2594
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Miller MR, Borthwick SJ, Shaw CA, McLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PWF, Newby DE (2009) Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspect 117:611–616
Moon D et al (2018) Altered proinflammatory cytokines and M1 polarization induced by PM2.5 in alveolar macrophages. Appl Ecol Environ Res 16:7699–7712. https://doi.org/10.15666/aeer/1606_76997712
Pieterse B, Felzel E, Winter R, Van Der Burg B, Brouwer A (2013) PAH-CALUX, an optimized bioassay for AhR-mediated hazard identification of polycyclic aromatic hydrocarbons (PAHs) as individual compounds and in complex mixtures. Environ Sci Technol 47:11651–11659
Prieto-Parra L, Yohannessen K, Brea C, Vidal D, Ubilla CA, Ruiz-Rudolph P (2017) Air pollution, PM2. 5 composition, source factors, and respiratory symptoms in asthmatic and nonasthmatic children in Santiago, Chile. Environ Int 101:190–200
Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, Stafoggia M, Samoli E, Weinmayr G, Dimakopoulou K, Nieuwenhuijsen M, Xun WW, Fischer P, Eriksen KT, Sørensen M, Tjønneland A, Ricceri F, de Hoogh K, Key T, Eeftens M, Peeters PH, Bueno-de-Mesquita HB, Meliefste K, Oftedal B, Schwarze PE, Nafstad P, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Penell J, de Faire U, Korek M, Pedersen N, Östenson CG, Pershagen G, Fratiglioni L, Concin H, Nagel G, Jaensch A, Ineichen A, Naccarati A, Katsoulis M, Trichpoulou A, Keuken M, Jedynska A, Kooter IM, Kukkonen J, Brunekreef B, Sokhi RS, Katsouyanni K, Vineis P (2016) Particulate matter air pollution components and risk for lung cancer. Environ Int 87:66–73
Radan M, Dianat M, Badavi M, Mard SA, Bayati V, Goudarzi G (2019) In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10): the effective role of gallic acid. Free Radic Res 53:210–225
Roh JS, Sohn DH (2018) Damage-associated molecular patterns in inflammatory diseases. Immune netw 18
Ruwanpura S, McLeod L, Anderson G, Jenkins B (2017) Novel role of inflammasomes in the molecular pathogenesis of emphysema. In: D97. Impact of inflammation on acute lung injury. American Thoracic Society, pp A7328-A7328
Sato A et al (2017) Diagnosis and clinical course of patients referred with chest X-ray abnormalities and suspected of nontuberculous mycobacterial lung disease. In: B62. Non-tuberculous mycobacteria: clinical aspects and cases. American Thoracic Society, pp A3966–A3966
Sharafkhaneh A, Hanania NA, Kim V (2008) Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 5:475–477
Strieter RM (2008) What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc 5:305–310
Taraseviciene-Stewart L, Voelkel NF (2008) Molecular pathogenesis of emphysema. J Clin Invest 118:394–402
Wang S et al (2017) Exposure to high level PM2. 5 causes increased central blood pressure. Circulation 136:A19823–A19823
Wilson M, Wynn T (2009) Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2:103–121
Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11:7–13
Wingate P, McAulay K, Anthony I, Crawford D (2009) Regulatory T cell activity in primary and persistent Epstein–Barr virus infection. J Med Virol 81:870–877
Wolters PJ, Collard HR, Jones KD (2014) Pathogenesis of idiopathic pulmonary fibrosis. Ann Rev Pathol Mech Dis 9:157–179
Zhang Y, Lang J, Cheng S, Li S, Zhou Y, Chen D, Zhang H, Wang H (2018) Chemical composition and sources of PM1 and PM2. 5 in Bei**g in autumn. Sci Total Environ 630:72–82
Zhao Y, Zhang H, Yang X, Zhang Y, Feng S, Yan X (2019) Fine particulate matter (PM2. 5) enhances airway hyperresponsiveness (AHR) by inducing necroptosis in BALB/c mice. Environ Toxicol Pharmacol 68:155–163
Acknowledgements
The authors are grateful to Dr. Kaveh Sadeghi at the Virology Department, School of Public Health, Tehran University of Medical Sciences, for scientific support.
Author information
Authors and Affiliations
Contributions
HRS and SR extracted data and wrote manuscript. MY designed the study and search strategy. BJ discussed the results.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shamsollahi, H.R., Jahanbin, B., Rafieian, S. et al. Particulates induced lung inflammation and its consequences in the development of restrictive and obstructive lung diseases: a systematic review. Environ Sci Pollut Res 28, 25035–25050 (2021). https://doi.org/10.1007/s11356-021-13559-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13559-5