Abstract
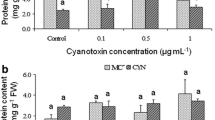
Toxic cyanobacterial blooms are often observed in freshwaters and may reflect the increased eutrophication of these environments and alterations in climate. Cyanotoxins, such as microcystins (MCs), are an effective threat to many life forms, ranging from plants to humans. Despite the research conducted to date on cyanotoxins, the risks associated to the use of contaminated water in agriculture require further elucidation. To tackle this aim, a research was conducted with the root-vegetable Daucus carota. The specific aims of this work were the following: (i) to evaluate the effects of MC-LR on the plant growth and photosynthesis; (ii) to evaluate the nutritional quality of carrot roots; and (iii) to measure bioaccumulation. To this purpose, young carrots were grown in soil during 1 month in natural conditions and exposed to Mycrocystis aeruginosa aqueous extracts containing environmentally realistic concentrations of MC-LR (10 and 50 MC-LR μg/L). The results showed that MC-LR may decrease root growth after 28 days of exposure to 50 μg/L and increase photosynthetic efficiency. We also observed changes in mineral and vitamin content in carrots as a result of the exposure to contaminated water. Moreover, MC-LR was detected in carrot roots by ELISA at very low concentration 5.23 ± 0.47 ng MC eq./g FW. The soil retained 52.7 % of the toxin potentially available for plants. This result could be attributed to MC-LR adsorption by soil particles or due to microbial degradation of the toxin. We conclude that the prolonged use of MC-LR-contaminated water may affect crop growth, alter the nutritional value of vegetable products, and potentiate contamination.




Similar content being viewed by others
References
Aboal M, Puig MA (2005) Intracellular and dissolved microcystin in reservoirs of the river Segura basin, Murcia, SE Spain. Toxicon 45(4):509–518. doi:10.1016/j.toxicon.2004.12.012
Ahamad MN, Saleemullah M, Shah HU, Khalil IA, Saljoqi AUR (2007) Determination of beta content in fresh vegetables using high performance liquid chromatography. Sarhad Journal of Agriculture 23(3):767–770
Azevedo CC, Azevedo J, Osório H, Vasconcelos V, Campos A (2014) Early physiological and biochemical responses of rice seedlings to low concentration of microcystin-LR. Ecotoxicology 23(2):107–121. doi:10.1007/s10646-013-1156-8
Bibo L, Yan G, Bangding X, Jiantong L, Yongding L (2008) A laboratory study on risk assessment of microcystin-RR in cropland. J Environ Manag 86(3):566–574. doi:10.1016/j.jenvman.2006.12.040
Bittencourt-Oliveira Mdo C, Cordeiro-Araújo MK, Chia MA, Arruda-Neto JD, de Oliveira ÊT, dos Santos F (2016) Lettuce irrigated with contaminated water: photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol Environ Saf 128:83–90. doi:10.1016/j.ecoenv.2016.02.014
Bláhová L, Adamovský O, Kubala L, Švihálková Šindlerová L, Zounková R, Bláha L (2013) The isolation and characterization of lipopolysaccharides from Microcystis aeruginosa, a prominent toxic water bloom forming cyanobacteria. Toxicon 76:187–196. doi:10.1016/j.toxicon.2013.10.011
Bourne DG, Riddles P, Jones GJ, Smith W, Blakeley RL (2001) Characterization of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin-LR. Environ Toxicol 16(6):523–534. doi:10.1002/tox.10013
Burýsková B, Hilscherová K, Babica P, Vrsková D, Marsálek B, Bláha L (2006) Toxicity of complex cyanobacterial samples and their fractions in Xenopus laevis embryos and the role of microcystins. Aquat Toxicol 80(4):346–354. doi:10.1016/j.aquatox.2006.10.001
Chen J, Song L, Dai J, Gan N, Zhili L (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidade of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43(4):393–400. doi:10.1016/j.toxicon.2004.01.011
Chen W, Song L, Gan N, Li L (2006) Sorption, degradation and mobility of microcystins in Chinese agriculture soils: risk assessment for groundwater protection. Environ Pollut 144(3):752–758. doi:10.1016/j.envpol.2006.02.023
Chen J, Dai J, Zhang H, Wang C, Zhou G, Han Z, Liu Z (2010) Bioaccumulation of microcystin and its oxidative stress in the apple (Malus pumila). Ecotoxicology 19(4):796–803. doi:10.1007/s10646-009-0456-5
Chen J, Han FX, Wang F, Zhang HQ, Shi ZQ (2012) Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotoxicol Environ Saf 76(2):193–199. doi:10.1016/j.ecoenv.2011.09.022
Corbel S, Mougin C, Bouaïcha N (2014a) Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 96:1–15. doi:10.1016/j.chemosphere.2013.07.056
Corbel S, Bouaïcha N, Mougin C (2014b) Dynamics of the toxic cyanobacterial microcystin-leucine-arginine peptide in agricultural soil. Environ Chem Lett 12(4):535–541. doi:10.1007/s10311-014-0482-2
Corbel S, Bouaïcha N, Nélieu S, Mougin C (2015) Soil irrigation with water and toxic cyanobacterial microcystins accelerates tomato development. Environ Chem Lett 13(4):447–452. doi:10.1007/s10311-015-0518-2
Crush JR, Briggs LR, Sprosen JM, Nichols SN (2008) Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ Toxicol 23(2):246–252. doi:10.1002/tox.20331
Dawson RM (1998) The toxicology of microcystins. Toxicon 36(7):953–962. doi:10.1016/S0041-0101(97)00102-5
Duy TN, Lam PKS, Shaw GR, Connell DW (2000) Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev Environ Contam Toxicol 163:113–186. doi:10.1007/978-1-4757-6429-1_3
El Khalloufi F, Oufdou K, Lahrouni M, El Ghazali I, Saqrane S, Vasconcelos V, Oudra B (2011) Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-rhizobia symbiosis. Ecotoxicol Environ Saf 74(3):431–438. doi:10.1016/j.ecoenv.2010.10.006
El Khalloufi F, El Ghazali I, Saqrane S, Oufdou K, Vasconcelos V, Oudra B (2012) Phytotoxic effects of a natural bloom extract containing microcystins on Lycopersicon esculentum. Ecotoxicol Environ Saf 79:199–205. doi:10.1016/j.ecoenv.2012.01.002
Figueiredo DR, Azeiteiro UM, Esteves SM, Gonçalves FJM, Pereira JM (2004) Microcystin-producing blooms—a serious global public health issue. Ecotoxicol Environ Saf 59(2):151–163. doi:10.1016/j.ecoenv.2004.04.006
Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B (2005) Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol 203:257–263. doi:10.1016/j.taap.2004.08.012
Freitas M, Azevedo J, Pinto E, Neves J, Campos A, Vasconcelos V (2015) Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol Environ Saf 116:59–67. doi:10.1016/j.ecoenv.2015.02.002
Fridovich I, Handler P (1961) Detection of free radicals generated during enzymic oxidation by the initiation of sulphite oxidation. J Biol Chem 236:1836–1840
Funari E, Testai E (2008) Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 38(2):97–125. doi:10.1080/10408440701749454
Gehringer MM, Kewada V, Coates N, Downing TG (2003) The use of Lepidium sativum in a plant bioassay system for the detection of microcystin-LR. Toxicon 41(7):871–876. doi:10.1016/S0041-0101(03)00049-7
Gurbuz F, Metcalf JS, Karahan AG, Codd GA (2009) Analysis of dissolved microcystins in surface water samples from Kovada Lake, Turkey. Sci Total Environ 407(13):4038–4046. doi:10.1016/j.scitotenv.2009.02.039
Hardy FJ, Johnson A, Hamel K, Preece E (2015) Cyanotoxin bioaccumulation in freshwater fish, Washington State, USA. Environ Monit Assess 187(11):667. doi:10.1007/s10661-015-4875-x
Hitzfeld BC, Höger SJ, Dietrich DR (2000) Cyanobacterial toxins: removal during drinking water treatment and human risk assessment. Environ Health Perspect 108(S1):113–122. doi:10.2307/3454636
Huang X, Chen L, Liu W, Qiao Q, Wu K, Wen J, Huang C, Tang R, Zhang X (2015) Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat Toxicol 165:41–50. doi:10.1016/j.aquatox.2015.05.009
IARC. (2010). Ingested nitrate and nitrite, and cyanobacterial peptide toxins, IARC Monogr Eval Carcinog Risks Hum Suppl, 94, Lyon, France. pp 327–412. ISSN 1014-711X. Available in: http://monographs.iarc.fr/ENG/Monographs/suppl7/suppl7.pdf
Ismail A, Sook Fun C (2003) Determination of vitamin C, β-carotene and riboflavin contents in five green vegetables organically and conventionally grown. Malays J Nutr 9(1):31–39
Järvenpää S, Lundberg-Niinistö C, Spoof L, Sjövall O, Tyystjärvi E, Meriluoto J (2007) Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography–mass spectrometry. Toxicon 49(6):865–874. doi:10.1016/j.toxicon.2006.12.008
Keutgen AJ, Pawelzic E (2007) Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J Agric Food Chem 55(10):4066–4072. doi:10.1021/jf070010k
Kótai, J. (1972). Instructions for preparation of modified nutrient solution Z8 for algae. Norwegian Institute for Water Research, B-11/69
Lahrouni M, Oufdou K, El Khalloufi F, Baz M, Lafuente A, Dary M, Pajuelo E, Oudra B (2013) Physiological and biochemical defense reactions of Vicia faba L.-rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ Sci Pollut Res Int 20(8):5405–5415. doi:10.1007/s11356-013-1535-y
Li TQ, Tao Q, Di ZZ, Lu F, Yang XE (2014) Effect of elevated CO2 concentration on photosynthetic characteristics of hyperaccumulator Sedum alfredii under cadmium stress. J Integr Plant Bio 57(7):653–660. doi:10.1111/jipb.12307
Manage PM, Edwards C, Singh BK, Lawton LA (2009) Isolation and identification of novel microcystin-degrading bacteria. Appl Environ Microbiol 75(21):6924–6928. doi:10.1128/AEM.01928-09
Maxwell K, Johnson N (2000) Cholorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668. doi:10.1093/jexbot/51.345.659
Mazid M, Khan TA, Khan ZH, Quddusi S, Mohammad F (2011) Occurrence, biosynthesis and potentialities of ascorbic acid in plants. Int. J. Pl.An and Env. Sci 1(2):167–184
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39(9):1411–1420. doi:10.1016/S0041-0101(01)00100-3
Metcalf JS, Beattie KA, Pflugmacher S, Codd GA (2000) Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol Lett 189(2):155–158. doi:10.1111/j.1574-6968.2000.tb09222.x
Mohamed Z, Al Shehri AM (2009) Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J Hazard Mater 172(1):310–315. doi:10.1016/j.jhazmat.2009.07.010
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. doi:10.1016/S0003-2670(00)88444-5
Nasri H, El Herry S, Bouaïcha N (2008) First reported case of turtle deaths during a toxic Microcystis spp. bloom in Lake Oubeira Algeria. Ecotoxicol Environ Saf 71(2):535–544. doi:10.1016/j.ecoenv.2007.12.009
Ni, W., Zhang, J., Luo Y. (2015). Microcystin accumulation in bighead carp (Aristichthys nobilis) during a Microcystis-dominated bloom and risk assessment of the dietary intake in a fish pond in China. Environ Sci Pollut Res Int:1–9. doi: 10.1007/s11356-015-4974-9
Noctor G, Foyer CH (1998) Ascorbate and glutathione. Kee** active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279. doi:10.1146/annurev.arplant.49.1.249
O’Neil JM, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14:313–334. doi:10.1016/j.hal.2011.10.027
Paerl HW, Paul VJ (2012) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363. doi:10.1016/j.watres.2011.08.002
Pereira S, Saker M, Vale M, Vasconcelos V (2009) Comparison of sensitivity of grasses (Lolium perenne L. and Festuca rubra L.) and lettuce (Lactuca sativa L.) exposed to water contaminated with microcystins. Bull Environ Contam Toxicol 83(1):81–84. doi:10.1007/s00128-009-9763-z
Pereira AL, Monteiro B, Azevedo J, Campos A, Osório H, Vasconcelos V (2015) Effects of the naturally-occurring contaminant microcystins on the Azolla filiculoides–Anabaena azollae symbiosis. Ecotoxicol Environ Saf 118:11–20. doi:10.1016/j.ecoenv.2015.04.008
Peuthert A, Chakrabarti S, Pflugmacher S (2007) Uptake of microcystins-LR and -LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ Toxicol 22:436–442. doi:10.1897/05-615R.1
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ Toxicol 17(4):407–413. doi:10.1002/tox.10071
Pflugmacher S, Wiegand C, Beattie KA, Krause E, Steinberg CEW, Codd GA (2001) Uptake, effects, and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (cav.) trin. ex steud. Environ Toxicol Chem 20(4):846–852. doi:10.1002/etc.5620200421
Pflugmacher S, Jung K, Lundvall L, Neumann S, Peuthert A (2006) Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ Toxicol Chem 25(9):2381–2387. doi:10.1897/05-615R.1
Pflugmacher S, Aulhorn M, Grimm B (2007a) Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol 175(3):482–489. doi:10.1111/j.1469-8137.2007.02144.x
Pflugmacher S, Hofmann J, Hübner B (2007b) Effects on growth and physiological parameters in wheat (Triticum aestivum L.) grown in soil and irrigated with cyanobacterial toxin contaminated water. Environ Toxicol Chem 26(12):2710–2716. doi:10.1897/07-145.1
Pichardo S, Pflugmacher S (2011) Study of the antioxidant response of several bean variants to irrigation with water containing MC-LR and cyanobacterial crude extract. Environ Toxicol 26(3):300–306. doi:10.1002/tox.20622
Pinheiro C, Azevedo J, Campos A, Loureiro S, Vasconcelos V (2013) Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 705(1):27–42. doi:10.1007/s10750-012-1372-x
Pinto E, Almeida AA, Aguiar AA, Ferreira IM (2014) Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: influence of soil composition. Food Chem 152:603–611. doi:10.1016/j.foodchem.2013.12.023
Prieto A, Campos A, Cameán A, Vasconcelos V (2011) Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol Environ Saf 74(7):1973–1980. doi:10.1016/j.ecoenv.2011.06.009
Ramanan S, Tang J, Velayudhan A (2000) Isolation and preparative purification of microcystin variants. J Chromatogr A 883(1–2):103–112. doi:10.1016/S0021-9673(00)00378-2
Rao PVL, Gupta N, Bhaskar ASB, Jayaraj R (2002) Toxins and bioactive compounds from cyanobacteria and their implications on human health. J Environ Biol 3(23):215–224
Romero-Oliva C-S, Contardo-Jara V, Block T, Pflugmacher S (2014) Accumulation of microcystin congeners in different aquatic plants and crops—a case study from lake Amatitlán, Guatemala. Ecotoxicol Environ Saf 102:121–128. doi:10.1016/j.ecoenv.2014.01.031
Sadler T, von Elert E (2014) Physiological interaction of Daphnia and Microcystis with regard to cyanobacterial secondary metabolites. Aquat Toxicol 156:96–105. doi:10.1016/j.aquatox.2014.08.003
Saqrane S, Ghazali IE, Oudra B, Bouarab L, Vasconcelos V (2008) Effect of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J Environ Sci Health B 43(5):443–451. doi:10.1080/10934520701796192
Saqrane S, Ouahid Y, El Ghazali I, Oudra B, Bouarab L, del Campo FF (2009) Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: a laboratory experimental approach. Toxicon 53(7–8):786–796. doi:10.1016/j.toxicon.2009.01.028
Singh DP, Beloy J, McInerney JK, Day L (2012) Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota). Food Chem 132(3):1161–1170. doi:10.1016/j.foodchem.2011.11.045
Spoof L, Vesterkvist P, Lindholm T, Meriluoto J (2003) Screening for cyanobacterial hepatotoxins, microcystins and nodularin in environmental water samples by reversed-phase liquid chromatography–electrospray ionisation mass spectrometry. J Chromatogr A 1020(1):105–119. doi:10.1016/S0021-9673(03)00428-X
Stüven J, Pflugmacher S (2007) Antioxidative stress response of Lepidium sativum due to exposure to cyanobacterial secondary metabolites. Toxicon 50(1):85–93. doi:10.1016/j.toxicon.2007.02.019
Trapp S. (2009). Bioaccumulation of polar and ionizable compounds in plants. Ecotoxicology Modeling, Emerging Topics in Ecotoxicology: Principles, Approaches and Perspectives 2, J. Devillers (ed.), Springer Science + Business Media, LLC. doi: 10.1007/978–1–4419-0197-2 11
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, Chen GC, Chen G, Yu SZ (1996) Detection of microcystins, a blue-green algal hepatotoxins, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17(6):1317–1321. doi:10.1093/carcin/17.6.1317
Vasconcelos VM (1995) Uptake and depuration of the heptapeptide toxin microcystin-LR in Mytilus galloprovincialis. Aquat Toxicol 32:227–237. doi:10.1016/0166-445X(94)00085-5
WHO (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. In: Bartram J (ed) Chorus I. London and New York, World Health
Yu SZ (1995) Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepatol 10(6):674–682. doi:10.1111/j.1440-1746.1995.tb01370.x
Zegura B, Straser A, Filipič M (2011) Genotoxicity and potential carcinogenicity of cyanobacterial toxins—a review. Mutat Res 727:16–41. doi:10.1016/j.mrrev.2011.01.002
Zhou L, Yu H, Chen K (2002) Relationship between microcystin in drinking water and colorectal cancer. Biomed Environ Sci 15(2):166–171
Zhou M, Tu WW, Xu J (2015) Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells. Toxicon 101:92–100. doi:10.1016/j.toxicon.2015.05.005
Acknowledgments
This research was partially supported by the European Regional Development Fund (ERDF) through the COMPETE-Operational Competitiveness Programme and national funds through FCT-Foundation for Science and Technology under the project PEst-C/MAR/LA0015/2013 and by Porto University under the framework of the project IJUP2011_3. A. Campos work is supported by a post-doctoral grant (SFRH/BPD/103683/2014) from FCT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(PDF 149 kb)
Rights and permissions
About this article
Cite this article
Machado, J., Azevedo, J., Freitas, M. et al. Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota . Environ Sci Pollut Res 24, 752–764 (2017). https://doi.org/10.1007/s11356-016-7822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7822-7