Abstract
Purpose
Maximal, safe resection of solid tumors is considered a critical first step in successful cancer treatment. The advent of fluorescence image-guided surgery (FIGS) using non-specific agents has improved patient outcomes, particularly in the case of glioblastoma. Molecularly targeted agents that recognize specific tumor biomarkers have the potential to augment these gains. Identification of the optimal combination of targeting moiety and fluorophore is needed prior to initiating clinical trials.
Procedures
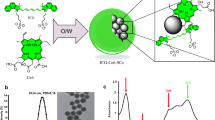
A 20-amino acid peptide (SBK2) recognizing the receptor protein-tyrosine phosphatase mu (PTPmu)–derived tumor-specific biomarker, with or without a linker, was conjugated to three different near-infrared fluorophores: indocyanine green (ICG), IRDye® 800CW, and Tide Fluor™ 8WS. The in vivo specificity, time course, and biodistribution were evaluated for each using mice with heterotopic human glioma tumors that express the PTPmu biomarker to identify component combinations with optimal properties for FIGS.
Results
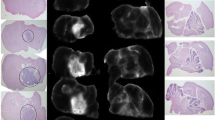
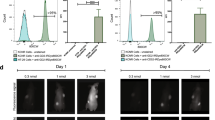
SBK2 conjugated to ICG demonstrated excellent specificity for gliomas in heterotopic tumors. SBK2-ICG showed significantly higher in vivo tumor labeling compared to the Scram-ICG control from 10 min to 24 h, p < 0.01 at all timepoints, following injection, as well as a significantly higher ex vivo tumor signal at 24 h, p < 0.001. Inserting a six-amino acid linker between the targeting peptide and ICG increased the clearance rate and resulted in significantly higher in vivo tumor signal relative to its linker-containing Scrambled control from 10 min to 8 h, p < 0.05 at all timepoints, after dosing. Agents made with the more hydrophilic IRDye® 800CW and Tide Fluor™ 8WS showed no specific tumor labeling relative to the controls. The IRDye 800CW-conjugated agents cleared within 1 h, while the non-specific fluorescent tumor signal generated by the Tide Fluor 8WS-conjugated agents persists beyond 24 h.
Conclusions
The SBK2 PTPmu-targeting peptide conjugated to ICG specifically labels heterotopic human gliomas grown in mice between 10 min and 24 h following injection. Similar molecules constructed with more hydrophilic dyes demonstrated no specificity. These studies present a promising candidate for use in FIGS of PTPmu biomarker–expressing tumors.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author (SBK) upon reasonable request.
References
Sim HW, Morgan ER, Mason WP (2018) Contemporary management of high-grade gliomas. CNS Oncol 7:51–65
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Youngblood MW, Stupp R, Sonabend AM (2021) Role of Resection in glioblastoma management. Neurosurg Clin N Am 32:9–22
Gorlia T, van den Bent MJ, Hegi ME et al (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol 9:29–38
Gittleman H, Cioffi G, Chunduru P et al (2019) An independently validated nomogram for isocitrate dehydrogenase-wild-type glioblastoma patient survival. Neuro-Oncol Adv 1:vdz007
Patil N, Somasundaram E, Waite KA et al (2021) Independently validated sex-specific nomograms for predicting survival in patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. J Neurooncol 155:363–372
Craig SEL, Wright J, Sloan AE, Brady-Kalnay SM (2016) Fluorescent-guided surgical resection of glioma with targeted molecular imaging agents: a literature review. World Neurosurg 90:154–163
Palmieri G, Cofano F, Salvati LF et al (2021) Fluorescence-guided surgery for high-grade gliomas: state of the art and new perspectives. Technol Cancer Res Treat 20:15330338211021605
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Jiang JX, Keating JJ, Jesus EM et al (2015) Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nuclear Med Mol Imaging 5:390–400
Stewart HL, Birch DJS (2021) Fluorescence guided surgery. Methods Appl Fluoresc 9(4):042002
Kaneko S, Kaneko S (2016) Fluorescence-guided resection of malignant glioma with 5-ALA. Int J Biomed Imaging 2016:6135293
Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME (2022) Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol 156:233–256
Landsman ML, Kwant G, Mook GA, Zijlstra WG (1976) Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol 40:575–583
Benson RC, Kues HA (1978) Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol 23:159–163
Jacques SL (2013) Optical properties of biological tissues: a review. Phys Med Biol 58:R37-61
Hong G, Antaris AL, Dai H (2017) Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng 1:0010
Zhang DY, Singhal S, Lee JYK (2019) Optical principles of fluorescence-guided brain tumor surgery: a practical primer for the neurosurgeon. Neurosurgery 85:312–324
Debie P, Hernot S (2019) Emerging fluorescent molecular tracers to guide intra-operative surgical decision-making. Front Pharmacol 10:510
Tanyi JL, Randall LM, Chambers SK et al (2023) A phase III study of pafolacianine injection (OTL38) for intraoperative imaging of folate receptor–positive ovarian cancer (Study 006). Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology 41:276–284. https://doi.org/10.1200/JCO.22.00291
Lui NS, Singhal S (2022) Intraoperative molecular imaging of lung cancer: a review. Surg Oncol Clin N Am 31:685–693
Van Keulen S, Hom M, White H, Rosenthal EL, Baik FM (2022) The evolution of fluorescence-guided surgery. Mol Imaging Biol. https://doi.org/10.1007/s11307-022-01772-8
Burden-Gulley SM, Gates TJ, Burgoyne AM et al (2010) A novel molecular diagnostic of glioblastomas: detection of an extracellular fragment of protein tyrosine phosphatase mu. Neoplasia (New York, NY) 12:305–316
Burgoyne AM, Phillips-Mason PJ, Burden-Gulley SM et al (2009) Proteolytic cleavage of protein tyrosine phosphatase mu regulates glioblastoma cell migration. Can Res 69:6960–6968
Burgoyne AM, Palomo JM, Phillips-Mason PJ et al (2009) PTPmu suppresses glioma cell migration and dispersal. Neuro Oncol 11:767–778
Phillips-Mason PJ, Craig SE, Brady-Kalnay SM (2014) A protease storm cleaves a cell-cell adhesion molecule in cancer: multiple proteases converge to regulate PTPmu in glioma cells. J Cell Biochem 115:1609–1623
Vincent J, Craig SEL, Johansen ML et al (2021) Detection of tumor-specific PTPmu in gynecological cancer and patient derived xenografts. Diagnostics (Basel, Switzerland) 11(2):181
Johansen ML, Gao Y, Hutnick MA et al (2017) Quantitative molecular imaging with a single Gd-based contrast agent reveals specific tumor binding and retention in vivo. Anal Chem 89:5932–5939
Johansen ML, Perera R, Abenojar E et al (2021) Ultrasound-based molecular imaging of tumors with PTPmu biomarker-targeted nanobubble contrast agents. Int J Mol Sci 22(4):1983
Covarrubias G, Johansen ML, Vincent J et al (2020) PTPmu-targeted nanoparticles label invasive pediatric and adult glioblastoma. Nanomed Nanotechnol Biol Med 28:102216
Herrmann K, Johansen ML, Craig SE et al (2015) Molecular imaging of tumors using a quantitative T 1 map** technique via magnetic resonance imaging. Diagnostics (Basel, Switzerland) 5:318–332
Burden-Gulley SM, Qutaish MQ, Sullivant KE et al (2011) Novel cryo-imaging of the glioma tumor microenvironment reveals migration and dispersal pathways in vivid three-dimensional detail. Can Res 71:5932–5940
Qutaish MQ, Sullivant KE, Burden-Gulley SM et al (2012) Cryo-image analysis of tumor cell migration, invasion, and dispersal in a mouse xenograft model of human glioblastoma multiforme. Mol Imag Biol 14:572–583
Burden-Gulley SM, Qutaish MQ, Sullivant KE et al (2013) Single cell molecular recognition of migrating and invading tumor cells using a targeted fluorescent probe to receptor PTPmu. Int J Cancer 132:1624–1632
Srinivasarao M, Galliford CV, Low PS (2015) Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discovery 14:203–219
Usama SM, Thapaliya ER, Luciano MP, Schnermann MJ (2021) Not so innocent: impact of fluorophore chemistry on the in vivo properties of bioconjugates. Curr Opin Chem Biol 63:38–45
Evers TH, van Dongen EM, Faesen AC, Meijer EW, Merkx M (2006) Quantitative understanding of the energy transfer between fluorescent proteins connected via flexible peptide linkers. Biochemistry 45:13183–13192
Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA (2016) Indocyanine green: historical context, current applications, and future considerations. Surg Innov 23:166–175
Renault K, Fredy JW, Renard PY, Sabot C (2018) Covalent modification of biomolecules through maleimide-based labeling strategies. Bioconjug Chem 29:2497–2513
Fontaine SD, Reid R, Robinson L, Ashley GW, Santi DV (2015) Long-term stabilization of maleimide-thiol conjugates. Bioconjug Chem 26:145–152
Desmettre T, Devoisselle JM, Mordon S (2000) Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol 45:15–27
Heintz R, Svensson CK, Stoeckel K, Powers GJ, Lalka D (1986) Indocyanine green: pharmacokinetics in the rabbit and relevant studies of its stability and purity. J Pharm Sci 75:398–402
DSouza AV, Lin H, Henderson ER, Samkoe KS, Pogue BW (2016) Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt 21:80901
Garcia M, Edmiston C, York T et al (2018) Bio-inspired imager improves sensitivity in near-infrared fluorescence image-guided surgery. Optica 5:413–422
Teng CW, Huang V, Arguelles GR et al (2021) Applications of indocyanine green in brain tumor surgery: review of clinical evidence and emerging technologies. Neurosurg Focus 50:E4
Starosolski Z, Bhavane R, Ghaghada KB, Vasudevan SA, Kaay A, Annapragada A (2017) Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS ONE 12:e0187563
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Huang R, Vider J, Kovar JL et al (2012) Integrin αvβ3-targeted IRDye 800CW near-infrared imaging of glioblastoma. Clin Cancer Res 18:5731–5740
Gong H, Kovar JL, Cheung L, Rosenthal EL, Olive DM (2014) A comparative study of affibody, panitumumab, and EGF for near-infrared fluorescence imaging of EGFR- and EGFRvIII-expressing tumors. Cancer Biol Ther 15:185–193
Acknowledgements
We thank Jennifer Major and Mamuni Swain for expert technical assistance, and Zoey Lockwood for assistance with the Varian spectrophotometers. An NIH shared instrument grant, 1S10RR031537-01, purchased the Orbitrap Elite LC-MS that was utilized for these agents in the Lerner Research Institute Proteomics Core at the Cleveland Clinic. SBK and AES were funded by a National Institutes of Health grant (R01CA217956). SBK was funded by the Tabitha Yee-May Lou Endowment Fund for Brain Cancer Research. Additional support was obtained from the National Institutes of Health sponsored Case Comprehensive Cancer Center, their Cancer Imaging Program and its cores (P30 CA043703).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Brady-Kalnay has licensed these agents to the biotechnology company NeoIndicate, where she serves as the Chief Scientific Officer. Mette Johansen is an inventor on the relevant patents.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johansen, M.L., Vincent, J., Rose, M. et al. Comparison of Near-Infrared Imaging Agents Targeting the PTPmu Tumor Biomarker. Mol Imaging Biol 25, 744–757 (2023). https://doi.org/10.1007/s11307-023-01799-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01799-5