Abstract
Purpose of the Report
Paediatric diffuse high-grade gliomas (PDHGG) are rare central nervous system neoplasms lacking effective therapeutic options. Molecular imaging of tumour metabolism might identify novel diagnostic/therapeutic targets. In this study, we evaluated the distribution and the dosimetry aspects of [64Cu]CuCl2 in PDHGG subjects, as copper is a key element in cellular metabolism whose turnover may be increased in tumour cells.
Material and Methods
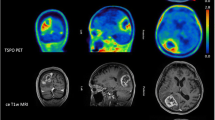
Paediatric patients with PDHGG were prospectively recruited. [64Cu]CuCl2 PET/CT was performed 1 h after tracer injection; if the scan was positive, it was repeated 24 and 72 h later. Lesion standardised uptake value (SUV) and target-to-background ratio (TBR) were calculated. Tumour and organ dosimetry were computed using the MIRD algorithm. Each patient underwent an MRI scan, including FLAIR, T2-weighted and post-contrast T1-weighted imaging.
Results
Ten patients were enrolled (median age 9, range 6–16 years, 6 females). Diagnoses were diffuse midline gliomas (n = 8, 5 of which with H3K27 alterations) and diffuse hemispheric gliomas (n = 2). Six patients had visible tracer uptake (SUV: 1.0 ± 0.6 TBR: 5 ± 3.1). [64Cu]CuCl2 accumulation was always concordant with MRI contrast enhancement and was higher in the presence of radiological signs of necrosis. SUV and TBR progressively increased on the 24- and 72-h acquisitions (p < 0.05 and p < 0.01, respectively). The liver and the abdominal organs received the highest non-target dose.
Conclusions
[64Cu]CuCl2 is a well-tolerated radiotracer with reasonably favourable dosimetric properties, showing selective uptake in tumour areas with visible contrast enhancement and necrosis, thus suggesting that blood–brain barrier damage is a pre-requisite for its distribution to the intracranial structures. Moreover, tracer uptake showed an accumulating trend over time. These characteristics could deserve further analysis, to determine whether this radiopharmaceutical might have a possible therapeutic role as well.




Similar content being viewed by others
References
Blionas A, Giakoumettis D, Klonou A et al (2018) Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med 6:251
Immanuel V, Kingsley PA, Negi P et al (2017) Variegated colors of pediatric glioblastoma multiforme: what to expect? Rare Tumors 9:6552
Rashed WM, Maher E, Adel M et al (2019) Pediatric diffuse intrinsic pontine glioma: where do we stand? Cancer Metastasis Rev 38:759–770
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251
Ansari M, Nasrolahi H, Kani AA et al (2012) Pediatric glioblastoma multiforme: a single-institution experience. Indian J Med Paediatr Oncol 33:155–160
Konar SK, Bir SC, Maiti TK et al (2017) A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord. J Neurosurg Pediatr 19:239–248
Boudaouara O, Charfi S, Bahri M et al (2019) Pediatric high grade gliomas: clinico-pathological profile, therapeutic approaches and factors affecting overall survival. Neurochirurgie 65:63–68
Grimm SA, Chamberlain MC (2013) Brainstem glioma: a review. Curr Neurol Neurosci Rep 13:346
Felker J, Broniscer A (2020) Improving long-term survival in diffuse intrinsic pontine glioma. Expert Rev Neurother 20:647–658
Covarrubias G, Johansen ML, Vincent J et al (2020) PTPmu-targeted nanoparticles label invasive pediatric and adult glioblastoma. Nanomedicine 28:102216
Lieberman NAP, DeGolier K, Kovar HM et al (2019) Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol 21:83–94
Shabason JE, Sutton D, Kenton O et al (2016) Patterns of failure for pediatric glioblastoma multiforme following radiation therapy. Pediatr Blood Cancer 63:1465–1467
Fangusaro J (2012) Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol 2:105
Cohen KJ, Pollack IF, Zhou T et al (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 13:317–323
Janssens GO, Jansen MH, Lauwers SJ et al (2013) Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 85:315–320
Janssens GO, Gandola L, Bolle S et al (2017) Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73:38–47
Janjua MB, Ban VS, El Ahmadieh TY et al (2020) Diffuse intrinsic pontine gliomas: diagnostic approach and treatment strategies. J Clin Neurosci 72:15–19
Katagi H, Louis N, Unruh D et al (2019) Radiosensitization by Histone H3 demethylase inhibition in diffuse intrinsic pontine glioma. Clin Cancer Res 25:5572–5583
Christensen M, Kamson DO, Snyder M et al (2014) Tryptophan PET-defined gross tumor volume offers better coverage of initial progression than standard MRI-based planning in glioblastoma patients. J Radiat Oncol 3:131–138
Law I, Albert NL, Arbizu J et al (2019) Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging 46:540–557
Huskisson E, Maggini S, Ruf M (2007) The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res 35:277–289
Horn D, Barrientos A (2008) Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 60:421–429
Brady DC, Crowe MS, Turski ML et al (2014) Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509:492–496
Ishida S, Andreux P, Poitry-Yamate C et al (2013) Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A 110:19507–19512
Shanbhag VC, Gudekar N, Jasmer K et al (2021) Copper metabolism as a unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res 1868:118893
Cui L, Gouw AM, LaGory EL et al (2021) Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat Biotechnol 39:357–367
Petruzzelli R, Polishchuk RS (2019) Activity and trafficking of copper-transporting ATPases in tumor development and defense against platinum-based drugs. Cells 8(9):1080
Williams HA, Robinson S, Julyan P et al (2005) A comparison of PET imaging characteristics of various copper radioisotopes. Eur J Nucl Med Mol Imaging 32:1473–1480
Bolzati C, Duatti A (2020) The emerging value of 64Cu for molecular imaging and therapy. Q J Nucl Med Mol Imaging 64:329–337
Bé MM, Cassette P, Lépy MC et al (2012) Standardization, decay data measurements and evaluation of 64Cu. Appl Radiat Isot 70:1894–1899
Righi S, Ugolini M, Bottoni G et al (2018) Biokinetic and dosimetric aspects of (64)CuCl(2) in human prostate cancer: possible theranostic implications. EJNMMI Res 8:18
Panichelli P, Villano C, Cistaro A et al (2016) Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm 31:159–167
McCarthy DW, Shefer RE, Klinkowstein RE et al (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43
ICRP (1995) Basic anatomical & physiological data for use in radiological protection: the skeleton: the Skeleton. ICRP Publication 70. Ann ICRP 25:1–80
Siegel JA, Thomas SR, Stubbs JB et al (1999) MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 40:37s–61s
Loeevinger R, Berman M (1968) A schema for absorbed-dose calculations for biologically-distributed radionuclides. J Nucl Med. Suppl 1:9–14
Snyder W, Ford, Warner GG, et al (1975) MIRD Pamphlet #11: S, absorbed dose per unit cumulated activity for selected radionuclides and organs
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
ICRP (2007) The 2007 Recommendations of the International Commission on Radiological Protection (2007) ICRP publication 103. Ann ICRP 37:1–332
ICRP (1991) 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 21:1–3
Cooney TM, Cohen KJ, Guimaraes CV et al (2020) Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21:e330–e336
Pérès EA, Toutain J, Paty LP et al (2019) (64)Cu-ATSM/(64)Cu-Cl(2) and their relationship to hypoxia in glioblastoma: a preclinical study. EJNMMI Res 9:114
Tateishi K, Tateishi U, Nakanowatari S et al (2014) (62)Cu-diacetyl-bis (N(4)-methylthiosemicarbazone) PET in human gliomas: comparative study with [(18)F]fluorodeoxyglucose and L-methyl-[(11)C]methionine PET. AJNR Am J Neuroradiol 35:278–284
Morana G, Piccardo A, Tortora D et al (2017) Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur J Nucl Med Mol Imaging 44:2084–2093
Morana G, Tortora D, Bottoni G et al (2020) Correlation of multimodal (18)F-DOPA PET and conventional MRI with treatment response and survival in children with diffuse intrinsic pontine gliomas. Theranostics 10:11881–11891
Piccardo A, Tortora D, Mascelli S et al (2019) Advanced MR imaging and (18)F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur J Nucl Med Mol Imaging 46:1685–1694
Grill J, Massimino M, Bouffet E et al (2018) Phase II, Open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol 36:951–958
Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E et al (2015) Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol 17:160–166
Thust S, Micallef C, Okuchi S et al (2021) Imaging characteristics of H3 K27M histone-mutant diffuse midline glioma in teenagers and adults. Quant Imaging Med Surg 11:43–56
Leach JL, Roebker J, Schafer A et al (2020) MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol 22:1647–1657
Colwell N, Larion M, Giles AJ et al (2017) Hypoxia in the glioblastoma microenvironment: sha** the phenotype of cancer stem-like cells. Neuro Oncol 19:887–896
Nobre L, Zapotocky M, Ramaswamy V, et al (2020) Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol 4:PO.19.00298
Chan PC, Lisco E, Lisco H et al (1976) The radiotoxicity of iodine-125 in mammalian cells II. A comparative study on cell survival and cytogenetic responses to 125IUdR, 131TUdR, and 3HTdR. Radiat Res 67:332–343
Kassis AI, Adelstein SJ, Haydock C et al (1982) Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res 90:362–373
Kassis AI, Sastry KS, Adelstein SJ (1985) Intracellular distribution and radiotoxicity of chromium-51 in mammalian cells: Auger-electron dosimetry. J Nucl Med 26:59–67
Piccardo A, Paparo F, Puntoni M et al (2018) (64)CuCl(2) PET/CT in prostate cancer relapse. J Nucl Med 59:444–451
Bohlken A, Cheung BB, Bell JL et al (2009) ATP7A is a novel target of retinoic acid receptor β2 in neuroblastoma cells. Br J Cancer 100:96–105
Parmar A, Pascali G, Voli F et al (2018) In vivo [64 Cu]CuCl 2 PET imaging reveals activity of Dextran-Catechin on tumor copper homeostasis. Theranostics 8:5645–5659
Kilari D, Icykowski KA, Pandya C et al (2016) copper transporter-CTR1 expression and pathological outcomes in platinum-treated muscle-invasive bladder cancer patients. Anticancer Res 36:495–501
Funding
This work was supported by the Italian Ministry of Health and by Regione Liguria “Ricerca Finalizzata di Rete NET-2019–12371188 GLI-HOPE”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanni Morana and Arnoldo Piccardo share senior co-authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiz, F., Bottoni, G., Ugolini, M. et al. Diagnostic and Dosimetry Features of [64Cu]CuCl2 in High-Grade Paediatric Infiltrative Gliomas. Mol Imaging Biol 25, 391–400 (2023). https://doi.org/10.1007/s11307-022-01769-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01769-3