Abstract
Purpose
Evaluation of [18F]fluoromisonidazole ([18F]FMISO)-positron emission tomography (PET) imaging as a metric for evaluating early response to trastuzumab therapy with histological validation in a murine model of HER2+ breast cancer.
Procedures
Mice with BT474, HER2+ tumors, were imaged with [18F]FMISO-PET during trastuzumab therapy. Pimonidazole staining was used to confirm hypoxia from imaging.
Results
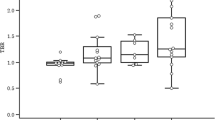
[18F]FMISO-PET indicated significant decreases in hypoxia beginning on day 3 (P < 0.01) prior to changes in tumor size. These results were confirmed with pimonidazole staining on day 7 (P < 0.01); additionally, there was a significant positive linear correlation between histology and PET imaging (r 2 = 0.85).
Conclusions
[18F]FMISO-PET is a clinically relevant modality which provides the opportunity to (1) predict response to HER2+ therapy before changes in tumor size and (2) identify decreases in hypoxia which has the potential to guide subsequent therapy.





Similar content being viewed by others
References
Hammond EM, Asselin MC, Forster D et al (2014) The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol) 26:277–288
Helmlinger G, Yuan F, Dellian M, Jain RK (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3:177–182
Gruber G, Greiner RH, Hlushchuk R et al (2004) Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res 6:R191–R198
Goel S, Duda DG, Xu L et al (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Mankoff DA, Dunnwald LK, Partridge SC, Specht JM (2009) Blood flow-metabolism mismatch: good for the tumor, bad for the patient. Clin Cancer Res 15:5294–5296
Hill RP, Stanley JA (1975) The response of hypoxic B16 melanoma cells to in vivo treatment with chemotherapeutic agents. Cancer Res 35:1147–1153
Teicher BA, Lazo JS, Sartorelli AC (1981) Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res 41:73–81
Generali D, Berruti A, Brizzi MP et al (2006) Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res 12:4562–4568
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274:113–126
Weber DC, Tille JC, Combescure C et al (2012) The prognostic value of expression of HIF1alpha, EGFR and VEGF-A, in localized prostate cancer for intermediate- and high-risk patients treated with radiation therapy with or without androgen deprivation therapy. Radiat Oncol 7:66
Society AC (2016) Cancer facts & figures 2016. American Cancer Society, Atlanta
Boekhout AH, Beijnen JH, Schellens JH (2011) Trastuzumab. Oncologist 16:800–810
Sorace AG, Quarles CC, Whisenant JG et al (2016) Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: preliminary results. Breast Cancer Res Treat 155:273–284
Izumi Y, Xu L, di Tomaso E et al (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416:279–280
Li X, Abramson RG, Arlinghaus LR et al (2015) Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Investig Radiol 50:195–204
Yankeelov TE, Abramson RG, Quarles CC (2014) Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol 11:670–680
Masaki Y, Shimizu Y, Yoshioka T et al (2015) The accumulation mechanism of the hypoxia imaging probe “FMISO” by imaging mass spectrometry: possible involvement of low-molecular metabolites. Sci Rep 5:16802
Muzi M, Peterson LM, O’Sullivan JN et al (2015) 18F-fluoromisonidazole quantification of hypoxia in human cancer patients using image-derived blood surrogate tissue reference regions. J Nucl Med 56:1223–1228
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 45:151–162
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Hatano T, Zhao S, Zhao Y et al (2013) Biological characteristics of intratumoral [F-18]fluoromisonidazole distribution in a rodent model of glioma. Int J Oncol 42:823–830
Cho H, Ackerstaff E, Carlin S et al (2009) Noninvasive multimodality imaging of the tumor microenvironment: registered dynamic magnetic resonance imaging and positron emission tomography studies of a preclinical tumor model of tumor hypoxia. Neoplasia 11:247–259, 242p following 259
Whisenant JG, Peterson TE, Fluckiger JU et al (2013) Reproducibility of static and dynamic (18)F-FDG, (18)F-FLT, and (18)F-FMISO MicroPET studies in a murine model of HER2+ breast cancer. Mol Imaging Biol 15:87–96
Oehler C, O’Donoghue JA, Russell J et al (2011) 18F-fluromisonidazole PET imaging as a biomarker for the response to 5,6-dimethylxanthenone-4-acetic acid in colorectal xenograft tumors. J Nucl Med 52:437–444
Arvold ND, Heidari P, Kunawudhi A et al (2016) Tumor hypoxia response after targeted therapy in EGFR-mutant non-small cell lung cancer: proof of concept for FMISO-PET. Technol Cancer Res Treat 15:234–242
Theze B, Bernards N, Beynel A et al (2015) Monitoring therapeutic efficacy of sunitinib using [(18)F]FDG and [(18)F]FMISO PET in an immunocompetent model of luminal B (HER2-positive)-type mammary carcinoma. BMC Cancer 15:534
Shah C, Miller TW, Wyatt SK et al (2009) Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin Cancer Res 15:4712–4721
Kramer-Marek G, Gijsen M, Kiesewetter DO et al (2012) Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med 53:629–637
Yang X, Knopp MV (2011) Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011:732848
Padhani AR (2002) Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging 16:407–422
Hudson JM, Williams R, Tremblay-Darveau C et al (2015) Dynamic contrast enhanced ultrasound for therapy monitoring. Eur J Radiol 84:1650–1657
Balleyguier C, Opolon P, Mathieu MC et al (2009) New potential and applications of contrast-enhanced ultrasound of the breast: own investigations and review of the literature. Eur J Radiol 69:14–23
Hardee ME, Eapen RJ, Rabbani ZN et al (2009) Her2/neu signaling blockade improves tumor oxygenation in a multifactorial fashion in Her2/neu + tumors. Cancer Chemother Pharmacol 63:219–228
Li XF, O’Donoghue JA (2008) Hypoxia in microscopic tumors. Cancer Lett 264:172–180
Cleeland CS, Allen JD, Roberts SA et al (2012) Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol 9:471–478
Acknowledgments
We thank the National Cancer Institute for support through U01CA174706, R01CA138599, R01 CA158079, U01CA142565, and 5T32CA093240. T.E.Y. is a CPRIT Scholar and the W.A. “Tex” Moncrief Professor of Computational Oncology at The University of Texas at Austin. We would like to thank Drs. Michael Freeman and Melinda Sanders for their help with this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sorace, A.G., Syed, A.K., Barnes, S.L. et al. Quantitative [18F]FMISO PET Imaging Shows Reduction of Hypoxia Following Trastuzumab in a Murine Model of HER2+ Breast Cancer. Mol Imaging Biol 19, 130–137 (2017). https://doi.org/10.1007/s11307-016-0994-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0994-1