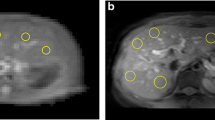
Abstract
Purpose
This study seeks to explore whether chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) can detect liver composition changes between after-meal and over-night-fast statuses.
Procedures
Fifteen healthy volunteers were scanned on a 3.0-T human MRI scanner in the evening 1.5–2 h after dinner and in the morning after over-night (12-h) fasting. Among them, seven volunteers were scanned twice to assess the scan–rescan reproducibility. Images were acquired at offsets (n = 41, increment = 0.25 ppm) from −5 to 5 ppm using a turbo spin echo (TSE) sequence with a continuous rectangular saturation pulse. Amide proton transfer-weighted (APTw) and GlycoCEST signals were quantified with the asymmetric magnetization transfer ratio (MTRasym) at 3.5 ppm and the total MTRasym integrated from 0.5 to 1.5 ppm from the corrected Z-spectrum, respectively. To explore scan time reduction, CEST images were reconstructed using 31 offsets (with 20 % time reduction) and 21 offsets (with 40 % time reduction), respectively.
Results
For reproducibility, GlycoCEST measurements in 41 offsets showed the smallest scan-rescan mean measurements variability, indicated by the lowest mean difference of −0.049 % (95 % limits of agreement, −0.209 to 0.111 %); for APTw, the smallest mean difference was found to be 0.112 % (95 % limits of agreement, −0.698 to 0.921 %) in 41 offsets. Compared with after-meal, both GlycoCEST measurement and APTw measurement under different offset number decreased after 12-h fasting. However, as the offsets number decreased (41 offsets vs. 31 offsets vs. 21 offsets), GlycoCEST map and APTw map became more heterogeneous and noisier.
Conclusion
Our results show that CEST liver imaging at 3.0 T has high sensitivity for fasting.






Similar content being viewed by others
References
Wallace K, Burt AD, Wright MC (2008) Liver fibrosis. Biochem J 411:1–18
Patel K, Shackel NA (2008) Current status of fibrosis markers. Curr Opin Gastroenterol 30:253–259
Anania C, Pacifico L, Ferraro F et al (2011) Aspartate transaminase to platelet ratio index (APRI) to assess liver fibrosis in patients with chronic liver disease. Hepat Mon 11:479–480
Tatsumi C, Kudo M, Ueshima K et al (2008) Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology 1(Suppl):27–33
Sy S, Huang S, Wang YX et al (2010) Terahertz spectroscopy of liver cirrhosis: investigating the origin of contrast. Phys Med Biol 55:7587–7596
Ligabue G, Besutti G, Scaglioni R et al (2013) MR quantitative biomarkers of non-alcoholic fatty liver disease: technical evolutions and future trends. Quant Imaging Med Surg 3:192–195
Janes CH, Lindor KD (1993) Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 118:96–98
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344:495–500
Zhou J, Lal B, Wilson DA et al (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Zhou J, Payen J, Wilson DA et al (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Yuan J, Chen S, King AD et al (2014) Amide proton transfer-weighted imaging of the head and neck at 3 T: a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed 27:1239–1247
Kim M, Chan Q, Anthony MP et al (2011) Assessment of glycosaminoglycan distribution in human lumbar intervertebral discs using chemical exchange saturation transfer at 3 T: feasibility and initial experience. NMR Biomed 24:1137–1144
Haneder S, Apprich SR, Schmitt B et al (2013) Assessment of glycosaminoglycan content in intervertebral discs using chemical exchange saturation transfer at 3.0 Tesla: preliminary results in patients with low-back pain. Eur Radiol 23:861–868
van Zijl PC, Jones CK, Ren J et al (2007) MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc Natl Acad Sci USA 104:4359–4364
Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H et al (2012) Magnetic resonance imaging of glutamate. Nat Med 18:302–306
Chan KW, McMahon MT, Kato Y et al (2012) Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 68:1764–1773
Walker-Samuel S, Ramasawmy R, Torrealdea F et al (2013) In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 19:1067–1072
Jones CK, Schlosser MJ, van Zijl PC et al (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592
Zhou J, Zhu H, Lim M et al (2013) Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128
Zhou J, Hong X, Zhao X et al (2013) APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med 70:320–327
Ren J, Trokowski R, Zhang S et al (2008) Imaging the tissue distribution of glucose in livers using a PARACEST sensor. Magn Reson Med 60:1047–1055
Sagiyama K, Zhang S, Dimitrov I et al (2014) In vivo monitoring of liver glycogen by chemical exchange saturation transfer imaging (GlycoCEST) in live mice. Proc Int Soc Magn Reson Med 22:0762
Bawden SJ, Mougin O, Hunter K et al (2014) Simultaneously measuring glycogen and lipid levels using localized CEST spectroscopy at 3T. Proc Intl Soc Mag Reson Med 22:3159
Deng M, Zhao F, Yuan J et al (2012) Liver T1ρ MRI measurement in healthy human subjects at 3 T: a preliminary study with a two-dimensional fast-field echo sequence. Br J Radiol 85:e590–e595
Ouwerkerk R, Pettigrew RI, Gharib AM (2012) Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology 265:565–575
Fleiss JL (1986) The design and analysis of clinical experiments. Wiley, New York, pp 1–31
Zhao F, Wang YX, Yuan J et al (2012) MR T1ρ as an imaging biomarker for monitoring liver injury progression and regression: an experimental study in rats with carbon tetrachloride intoxication. Eur Radiol 22:1709–1716
Zhao F, Yuan J, Deng M et al (2013) Further exploration of MRI techniques for liver T1rho quantification. Quant Imaging Med Surg 3:308–315
Wang YX, Yuan J, Chu ES et al (2011) T1rho MR imaging is sensitive to evaluate liver fibrosis: an experimental study in a rat biliary duct ligation model. Radiology 259:712–719
Wang YX, Yuan J (2014) Evaluation of liver fibrosis with T1ρ MR imaging. Quant Imaging Med Surg 4:152–155
Allkemper T, Sagmeister F, Cicinnati V et al (2014) Evaluation of fibrotic liver disease with whole-liver T1ρ MR imaging: a feasibility study at 1.5 T. Radiology 271:408–415
Mannelli L, Wilson GJ, Dubinsky TJ et al (2012) Assessment of the liver strain among cirrhotic and normal livers using tagged MRI. J Magn Reson Imaging 36:1490–1495
Salameh N, Larrat B, Abarca-Quinones J et al (2009) Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 253:90–97
Bonekamp S, Kamel I, Solga S, Clark J (2009) Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol 50:17–35
Lu PX, Huang H, Yuan J et al (2014) Decreases in molecular diffusion, perfusion fraction and perfusion-related diffusion in fibrotic livers: a prospective clinical intravoxel incoherent motion MR imaging study. PLoS One 9, e113846
Yoon JH, Lee JM, Baek JH et al (2014) Evaluation of hepatic fibrosis using intravoxel incoherent motion in diffusion-weighted liver MRI. J Comput Assist Tomogr 38:110–116
Jones CK, Huang A, Xu J et al (2013) Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage 77:114–124
** T, Wang P, Zong X, Kim SG (2013) MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear over hauser effect at 9.4 T. Magn Reson Med 69(3):760–770
Heo HY, Zhang Y, Lee DH et al (2015) Quantitative assessment of amide proton transfer (APT) and nuclear Overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: application to a rat glioma model at 4.7 T. Magn Reson Med. doi:10.1002/mrm.25581
Lu J, Zhou J, Cai C et al (2015) Observation of true and pseudo NOE signals using CEST-MRI and CEST-MRS sequences with and without lipid suppression. Magn Reson Med 73:1615–1622
Pagadala M, Kasumov T, McCullough AJ et al (2012) Role of ceramides in nanalcoholic fatty liver disease. Trends Endocrinol Metab 23:365–371
Li L, Tang X, Taylor KG et al (2002) Conformational characterization of ceramides by nuclear magnetic resonance spectroscopy. Biophys J 82:2067–2080
Zhao F, Deng M, Yuan J et al (2012) Experimental evaluation of accelerated T1rho relaxation quantification in human liver using limited spin-lock times. Korean J Radiol 13:736–742
Wang YX, Zhao F, Yuan J et al (2013) Accelerated T1rho relaxation quantification in intervertebral disc using limited spin-lock times. Quant Imaging Med Surg 3:54–58
Yuan J, Zhao F, Griffith JF et al (2012) Optimized efficient liver T(1ρ) map** using limited spin lock times. Phys Med Biol 57:1631–1640
Zhu Y, Zhang Q, Liu Q et al (2015) PANDA-T1ρ: integrating principal component analysis and dictionary learning for fast T1ρ map**. Magn Reson Med 37:263–272
Kim M, Gillen J, Landman BA et al (2009) Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 61:1441–1450
Sun PZ, Benner T, Kumar A, Sorensen AG (2008) Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn Reson Med 60:834–841
Zaiss M, Schmitt B, Bachert P (2011) Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson 211:149–155
Zaiss M, Bachert P (2013) Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys Med Biol 58:R221–R269
Pang Y, Jiang X, Zhang X (2014) Sparse parallel transmission on randomly perturbed spiral k-space trajectory. Quant Imaging Med Surg 4:106–111
Pang Y, Yu B, Zhang X (2014) Enhancement of the low resolution image quality using randomly sampled data for multi-slice MR imaging. Quant Imaging Med Surg 4:136–144
Acknowledgments
This study was partially by grants from the Research Grants Council of the Hong Kong SAR (Project No. 476313 and Project No. SEG_CUHK02) and the National Institutes of Health (R01EB009731, R01CA166171).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, M., Chen, SZ., Yuan, J. et al. Chemical Exchange Saturation Transfer (CEST) MR Technique for Liver Imaging at 3.0 Tesla: an Evaluation of Different Offset Number and an After-Meal and Over-Night-Fast Comparison. Mol Imaging Biol 18, 274–282 (2016). https://doi.org/10.1007/s11307-015-0887-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0887-8