Abstract
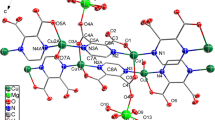
Dioxidomolybdenum(VI) complex, [MoVIO2(HL)2] (1) using ligand H2L (I) (synthesised from o-phenylenediamine and salicylaldehyde) and it’s heterogenized form [MoVIO2(L)2]@PS (2), supported on chloromethylated polystyrene (PS) have been isolated. The homogenous complex has been characterized by various spectroscopic techniques (FT-IR, UV–Visible, 1H and 13C NMR), elemental analysis (C, H and N), single crystal X-ray and thermal studies. The heterogeneous compound 2 was additionally studied by field emission-scanning electron microscopy, energy dispersive spectroscopy, UV–Visible diffuse reflectance spectroscopy and microwave plasma atomic emission spectroscopy. The reactivity of compounds 1 and 2 was studied towards their peroxidase mimetic activity in the oxidation of dopamine to aminochrome driven by H2O2 as an oxidant. Kinetic studies show that the reaction follows Michaelis–Menten like kinetics in both cases. Heterogenized compound 2 was further used to synthesize neuromelanin, a form of polymer generally observed in brain cells upon oxidation of dopamine and is a probable cause of Parkinson’s disease.
Graphical Abstract
Dioxidomolybdenum(VI) complex, [MoVIO2(HL)2] (1) using ligand H2L (I) (synthesised from o-phenylenediamine and salicylaldehyde) has been immobilized on chloromethylated polystyrene and used as catalyst for the peroxidase mimetic activity in the oxidation of dopamine to aminochrome driven by H2O2 as an oxidant.



















Similar content being viewed by others
Data Availability
The data that support the findings of this study are mostly provided in the research article.
References
Yan MH, Wang X, Zhu X (2013) Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radical Biol Med 62:90–101. https://doi.org/10.1016/j.freeradbiomed.2012.11.014
Gligorich KM, Sigman MS (2009) Recent advancements and challenges of palladiumII-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem Commun 2009(26):3854–3867. https://doi.org/10.1039/B902868D
Piera J, Backvall JE (2008) Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—a biomimetic approach. Angew Chem Int Ed 47:3506–3523. https://doi.org/10.1002/anie.200700604
Stahl SS (2005) Palladium-catalyzed oxidation of organic chemicals with O2. Science 309:1824–1820. https://doi.org/10.1126/science.1114666
Punniyamurthy T, Velusamy S, Iqbal J (2005) Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem Rev 105:2329–2364. https://doi.org/10.1021/cr050523v
Stahl SS (2004) Palladium oxidase catalysis: selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew Chem Int Ed 43:3400–3420. https://doi.org/10.1002/anie.200300630
Funabiki T (1997) Iron model studies on dioxygenases. Springer, Berlin
Osborne RL, Klinman JP (2011) Copper–oxygen chemistry. Wiley, Hoboken, pp 1–22
Bertini I, Gray HB, Lippard SJ, Valentine JS (1994) Bioinorganic chemistry. University Science Books, Sausalito
Martell AE, Sawyer DT (1988) Oxygen complexes and oxygen activation by transition metals. Springer, Boston, pp 131–148. https://doi.org/10.1007/978-1-4613-0955-0
Maurya MR, Dhaka S, Avecilla F (2015) Oxidative bromination of monoterpene (thymol) using dioxidomolybdenum(VI) complexes of hydrazones of 8-formyl-7-hydroxy-4-methylcoumarin. Polyhedron 96:79–87. https://doi.org/10.1016/j.poly.2015.05.001
Maurya MR, Rana L, Avecilla F (2016) Oxidoperoxidotungsten(VI) and dioxidotungsten(VI) complexes catalyzed oxidative bromination of thymol in presence of H2O2–KBr–HClO4. Inorg Chim Acta 440:172–180. https://doi.org/10.1016/j.ica.2015.10.045
Maurya MR, Rana L, Avecilla F (2017) Molybdenum complexes with a µ-O{MoO2}2 core: their synthesis, crystal structure and application as catalysts for the oxidation of bicyclic alcohols using N-based additives. New J Chem 41:724–734. https://doi.org/10.1039/C6NJ03162E
Maurya MR, Saini N, Avecilla F (2015) Effect of N-based additive on the optimization of liquid phase oxidation of bicyclic, cyclic and aromatic alcohols catalyzed by dioxidomolybdenum(VI) and oxidoperoxidomolybdenum(VI) complexes. RSC Adv 5:101076–101088. https://doi.org/10.1039/C5RA16490G
Siu TC, Silva I, Lunn MJ, John A (2020) Influence of the pendant arm in deoxydehydration catalyzed by dioxomolybdenum complexes supported by amine bisphenolate ligands. New J Chem 44:9933–9941. https://doi.org/10.1039/D0NJ02151B
Riisiö A, Lehtonen A, Hänninen MM, Sillanpää R (2013) Synthesis, structure and catalytic properties of dinuclear MoVI complexes with ditopic diaminotetraphenols. Eur J Inorg Chem 2013:1499–1508. https://doi.org/10.1002/ejic.201201234
Maurya MR, Arya A, Adäo P, Pessoa JC (2008) Immobilisation of oxovanadium(IV), dioxomolybdenum(VI) and copper(II) complexes on polymers for the oxidation of styrene, cyclohexene and ethylbenzene. Appl Catal A 351:239–252. https://doi.org/10.1016/j.apcata.2008.09.021
Maurya MR, Kumar N (2015) Sodium bicarbonate assisted oxidation, by H2O2, of styrene and cyclohexene using polymer grafted dioxidomolybdenum(VI) complex as a catalyst. J Mol Catal A 406:204–212. https://doi.org/10.1016/j.molcata.2015.06.002
Maurya MR, Tomar R, Rana L, Avecilla F (2018) Trinuclear dioxidomolybdenum(VI) complexes of tritopic phloroglucinol-based ligands and their catalytic applications for the selective epoxidation of olefins. Eur J Inorg Chem 2018:2952–2964. https://doi.org/10.1002/ejic.201800440
Wong YL, Tong LH, Dilworth JR, Ng DKP, Lee HK (2010) New dioxo–molybdenum(VI) and –tungsten(VI) complexes with N-capped tripodal N2O2 tetradentate ligands: synthesis, structures and catalytic activities towards olefinepoxidation. Dalton Trans 39:4602–4611. https://doi.org/10.1039/B926864B
Dupé A, Hossain MK, Schachner JA, Belaj F, Lehtonen A, Nordlander E, Mösch-Zanetti NC (2015) Dioxomolybdenum(VI) and -tungsten(VI) complexes with multidentate aminobisphenol ligands as catalysts for olefin epoxidation. Eur J Inorg Chem 2015:3572–3579. https://doi.org/10.1002/ejic.201500055
Annese C, Caputo D, D′Accolti L, Fusco C, Nacci A, Rossin A, Tuci G, Giambastiani G (2019) Dioxomolybdenum(VI) complexes with salicylamide ligands: synthesis, structure, and catalysis in the epoxidation of olefins under eco-friendly conditions. Eur J Inorg Chem 2019(2):221–229. https://doi.org/10.1002/ejic.201801096
Hossain MK, Köhntopp A, Haukka M, Richmond MG, Lehtonen A, Nordlander E (2020) Cis- and trans molybdenum oxo complexes of a prochiral tetradentate aminophenolate ligand: synthesis, characterization and oxotransfer activity. Polyhedron 178:114312. https://doi.org/10.1016/j.poly.2019.114312
Ta S, Ghosh M, Salam N, Ghosh S, Brandão P, Félix V, Hira SK, Manna PP, Das D (2019) Mo(VI) complexes of amide–imine conjugates for tuning the selectivity of fluorescence recognition of Y(III) vs. pb(II). ACS Appl Bio Mater 2:3964–3973. https://doi.org/10.1039/D2RA06035C
Ziegler JE, Du G, Fanwick PE, Abu-Omar MM (2009) An efficient method for the preparation of oxo molybdenum salalen complexes and their unusual use as hydrosilylation catalysts. Inorg Chem 48:11290–11296. https://doi.org/10.1021/ic901794h
Maurya MR, Saini N, Avecilla F (2016) Study of temperature dependent three component dynamic covalent assembly via Hantzsch reaction catalyzed by dioxido- and oxidoperoxidomolybdenum(VI) complexes under solvent free conditions. RSC Adv 6:12993–13009. https://doi.org/10.1039/C5RA24791H
Maurya MR, Rana L, Jangra N, Avecilla F (2017) Bis{cis-[MoO2]} complexes of 4,6-diacetyl resorcinol bis(hydrazone) and their catalytic application for the three components dynamic covalent assembly via Hantzsch reaction. ChemistrySelect 2:6767–6777. https://doi.org/10.1002/slct.201701629
Maurya MR, Tomar R, Gupta P, Avecilla F (2020) Trinuclear cis-dioxidomolybdenum(VI) complexes of compartmental C3 symmetric ligands: synthesis, characterization, DFT study and catalytic application for hydropyridines (Hps) via the Hantzsch reaction. Polyhedron 186:114617. https://doi.org/10.1016/j.poly.2020.114617
Muthusami R, Moorthy M, Irena K, Govindaraj A, Manickam C, Rangappan R (2018) Designing a biomimetic catalyst for phenoxazinone synthase activity using a mesoporous Schiff base copper complex with a novel double-helix morphology. New J Chem 42:18608–18620. https://doi.org/10.1039/C8NJ03638A
Maia DO, Chagas AMS, Araújo AMM, Júnior AVM, Ferreira IML, Lemos FCD, Gondim AD (2018) Catalytic pyrolysis of glycerol in the presence of Nickel (II) Schiff base complex supported in SBA-15: kinetic and products (TG-FTIR and PY-CG/MS). Thermochim Acta 669:160–168. https://doi.org/10.1016/j.tca.2018.09.005
Heydari N, Bikas R, Shaterian M, Lis T (2022) Green solvent free epoxidation of olefins by a heterogenised hydrazone-dioxidotungsten(VI) coordination compound. RSC Adv 12:4813–4827. https://doi.org/10.1039/d1ra09217k
Heydari N, Bikas R, Shaterian M, Krawczyk MS, Lis T (2023) Investigation of the substituent effects on the oxidation of styrene derivatives by silica-supported heterogeneous oxidovanadium(V) coordination compound. Appl Organomet Chem 37:e6976. https://doi.org/10.1002/aoc.6976
Heydari N, Bikas R, Shaterian M, Lis T (2023) Selective oxidation of benzyl alcohols by silica-supported heterogeneous catalyst containing dioxidotungsten(VI) core. Appl Organomet Chem 37:e6939. https://doi.org/10.1002/aoc.6939
Mirzaee M, Bahramian B, Shahraki M, Moghadam H, Mirzaee A (2018) Molybdenum containing catalysts grafted on functionalized hydrous zirconia nano-particles for epoxidation of alkenes. Catal Lett 148:3003–3017. https://doi.org/10.1007/s10562-018-2521-2
Maurya MR, Chauhan A, Verma A, Kumar U, Avecilla F (2022) Amine-functionalized titanium dioxide supported dioxidomolybdenum(VI) complexes as functional model for phenoxazinone synthase enzyme. Catal Today 388–389:274–287. https://doi.org/10.1016/j.cattod.2020.06.031
Maurya MR, Chauhan A, Avecilla F (2022) Synthesis, characterization and biomimetic activity of heterogenized dioxidomolybdenum(VI) and analogous homogeneous complexes. ChemistrySelect 7:e202202327. https://doi.org/10.1002/slct.202202327
Maurya MR, Chauhan A (2023) Synthesis, characterization and biomimetic activity of heterogenized dioxidomolybdenum(VI) complex and its homogeneous analogue. Top Catal 66:420–434. https://doi.org/10.1007/s11244-022-01747-7
Maurya MR, Chauhan A (2023) Titania supported dioxidotungsten(VI) complex as bio-mimic for the type II copper-containing oxidase enzyme phenoxazinone synthase. New J Chem 47:2858–2873. https://doi.org/10.1039/d2nj05277f
Hadigavabar AD, Tabatabaeian K, Zanjanchi MA, Mamaghani M (2018) Molybdenum anchored onto zeolite beta: an efficient catalyst for the one-pot synthesis of octahydroquinazolinone derivatives under solvent-free conditions. React Kinet Mech Catal 124:857–871. https://doi.org/10.1007/s11144-018-1370-8
Maurya MR (2018) Vanadium complexes based polymer supported catalysts: a brief account of research from our group. Top Catal 61:1500–1513. https://doi.org/10.1007/s11244-018-1006-2
Maurya MR, Chauhan A, Arora S, Gupta P (2022) Triazole based oxidovanadium(V) complex supported on chloromethylated polymer and its catalytic activity for the synthesis of dihydropyrimidinones (DHPMs). Catal Today 397–399:3–15. https://doi.org/10.1016/j.cattod.2022.03.006
Mungse HP, Verma S, Kumar N, Sain B, Khatri OP (2012) Grafting of oxo-vanadium Schiff base on graphenenanosheets and its catalytic activity for the oxidation of alcohols. J Mater Chem 22:5427–5433. https://doi.org/10.1039/C2JM15644J
Masteri-Farahani M, Ghahremani M (2019) Surface functionalization of graphene oxide and graphene oxide-magnetite nanocomposite with molybdenum-bidentate Schiff base complex. J Phys Chem Solids 130:6–12. https://doi.org/10.1016/j.jpcs.2019.02.006
Majumdar A, Sarkar S (2011) Bioinorganic chemistry of molybdenum and tungsten enzymes: a structural–functional modeling approach. Coord Chem Rev 255:1039–1054. https://doi.org/10.1016/j.ccr.2010.11.027
Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 282:15597–15605. https://doi.org/10.1074/jbc.m610893200
Double KL, Zecca L, Costi P, Mauer M, Griesinger C, Ito S, Ben-Shachar D, Bringmann G, Fariello RG, Riederer P, Gerlach M (2000) Structural characteristics of human substantia nigra neuromelanin and synthetic dopamine melanins. J Neurochem 75:2583–2589. https://doi.org/10.1046/j.1471-4159.2000.0752583.x
Napolitano A, Manini P, d’Ischia M (2011) Oxidation chemistry of catecholamines and neuronal degeneration: an update. Curr Med Chem 18:1832–1845. https://doi.org/10.2174/092986711795496863
Hastings TG (1995) Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem 64(2):919–924. https://doi.org/10.1046/j.1471-4159.1995.64020919.x
Segura-Aguilar J (1996) Peroxidase activity of liver microsomal vitamin D 25-hydroxylase catalyzes 25-hydroxylation of vitamin D3 and oxidation of dopamine to aminochrome. Biochem Mol Med 58(1):122–129. https://doi.org/10.1006/bmme.1996.0039
Foppoli C, Coccia R, Cini C, Rosei MA (1997) Catecholamines oxidation by xanthine oxidase. Biochim Biophys Acta 1334(2–3):200–206. https://doi.org/10.1016/s0304-4165(96)00093-1
Galzigna L, Iuliis AD, Zanatta L (2000) Enzymatic dopamine peroxidation in substantia nigra of human brain. Clin Chim Acta 300(1–2):131–138. https://doi.org/10.1016/S0009-8981(00)00313-2
Thompson CM, Capdevila JH, Strobel HW (2000) Recombinant cytochrome P450 2D18 metabolism of dopamine and arachidonic acid. J Pharmacol Exp Ther 294(3):1120–1130
Segura-Aguilar J, Muñoz P, Paris I (2016) Aminochrome as new preclinical model to find new pharmacological treatment that stop the development of Parkinson’s disease. Curr Med Chem 23:346–359. https://doi.org/10.2174/0929867323666151223094103
Segura-Aguilar J (2019) On the role of aminochrome in mitochondrial dysfunction and endoplasmic reticulum stress in Parkinson’s disease. Front Neurosci 13:271. https://doi.org/10.3389/fnins.2019.00271
Herrera A, Muñoz P, Steinbusch HWM, Segura-Aguilar J (2017) Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson’s disease? ACS Chem Neurosci 8:702–711. https://doi.org/10.1021/acschemneuro.7b00034
Chen GJJ, McDonald JW, Newton WE (1976) Synthesis of Mo(IV) and Mo(V) complexes using oxo abstraction by phosphines. Mechanistic implications. Inorg Chem 15:2612–2615. https://pubs.acs.org/doi/pdf/https://doi.org/10.1021/ic50165a008
Rao NS, Ratnam CV (1955) Reaction between o-phenylenediamine and aromatic aldehydes. Curr Sci 24:299–301. https://www.jstor.org/stable/24055218
Maurya MR, Tomar R, Avecilla F, Ribeiro N, Carvalho MFN, Kuznetsov ML, Correia I, Pessoa JC (2020) Trinuclear vanadium(IV) and vanadium(V) complexes derived from 2,4,6-triacetylphloroglucinol and study of their peroxidase mimicking activity. Dalton Trans 49:2589–2609. https://doi.org/10.1039/C9DT04415A
Peuronen A, Sillanpää R, Lehtonen A (2018) The synthesis and vibrational spectra of 16O-enriched and 18O-enriched cis-MO2 (M = Mo, W) complexes. ChemistrySelect 3:3814–3818. https://doi.org/10.1002/slct.201800671
Bridelli MG, Tampellini D, Zecca L (1999) The structure of neuromelanin and its iron binding site studied by infrared spectroscopy. FEBS Lett 457:18–22. https://doi.org/10.1016/S0014-5793(99)01001-7
Zecca L, Pietra R, Goj C, Mecacci C, Radice D, Sabbioni E (1994) Iron and other metals in neuromelanin, substantia nigra, and putamen of human brain. J Neurochem 62:1097–1101. https://doi.org/10.1046/j.1471-4159.1994.62031097.x
Smythies J (1996) On the function of neuromelanin. Proc Royal Soc Lond Ser B Biol Sci 263:487–489
Maurya MR, Uprety B, Avecilla F, Adão P, Pessoa JC (2015) Vanadium(V) complexes of a tripodal ligand, their characterisation and biological implications. Dalton Trans 44:17736–17755. https://doi.org/10.1039/C5DT02716K
Oliveira JAF, Silva MP, Souza B, Camargo TP, Szpoganicz B, Neves A, Bortoluzzi A (2016) Dopamine polymerization promoted by a catecholase biomimetic CuII(µ-OH)CuII complex containing a triazine-based ligand. Dalton Trans 45:15294–15297. https://doi.org/10.1039/C6DT02032A
Albada HB, Golub E, Willner I (2016) Rational design of supramolecular hemin/G-quadruplex–dopamine aptamer nucleoapzyme systems with superior catalytic performance. Chem Sci 7:3092–3101. https://doi.org/10.1039/C5SC04832J
Biniuri Y, Albada B, Wolff M, Golub E, Gelman D, Willner I (2018) Cu2+ or Fe3+ terpyridine/aptamer conjugates: nucleoapzymes catalyzing the oxidation of dopamine to aminochrome. ACS Catal 8:1802–1809. https://doi.org/10.1021/acscatal.7b03454
Golub E, Albada HB, Liao WC, Biniuri Y, Willner I (2016) Nucleoapzymes: hemin/G-quadruplex DNAzyme–aptamer binding site conjugates with superior enzyme-like catalytic functions. J Am Chem Soc 138:164–172. https://doi.org/10.1021/jacs.5b09457
Acknowledgements
M. R. M. thanks the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India, New Delhi, India for financial support of the work (Grant number CRG/2018/ 000182). A.P. thanks Council of Scientific and Industrial Research, New Delhi, India for Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, M.R., Patter, A., Chauhan, A. et al. Dioxidomolybdenum(VI) Complex Supported on Chloromethylated Polymer and Its Catalytic Role in Peroxidase Mimicking Activity Towards Oxidation of Dopamine. Top Catal 67, 466–482 (2024). https://doi.org/10.1007/s11244-023-01861-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01861-0