Abstract
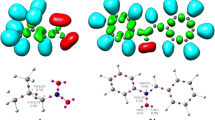
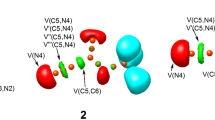
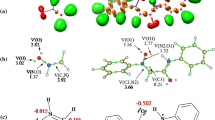
The intramolecular [3 + 2] cycloaddition (32CA) reactions of allenic nitrones have been studied within the molecular electron density theory (MEDT) at the MPWB1K/6-311G(d,p) computational level. These zwitter-ionic type 32CA reactions show high activation free energies between 22.2 and 34.9 kcal mol−1 in ethanol consistent with their predicted non-polar character and follow one-step mechanism with highly asynchronous transition states. Interestingly, when the nitrone and the allene moieties are separated by two methylene units, the [3 + 2] addition is energetically feasible along the C5-C6 terminal double bond of the allene, while the presence of four methylene units change the cyclization selectivity towards the internal C4-C5 double bond of the allene. This is in complete agreement with the experimental outcomes. The molecular mechanism study in terms of bonding evolution theory (BET) shows varied electron density changes along these two reaction paths. Finally, the topological analysis of AIM (atoms-in-molecules) reveals the presence of non-covalent interactions at the interatomic bonding regions of the transition states, which agrees well with the electron localization function analysis and the forming C–C and C-O bond distances.













Similar content being viewed by others
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Pinho e Melo TM (2009) Allenes as dipolarophiles and 1,3-dipole precursors: synthesis of carbocyclic and heterocyclic compounds. Curr Org Chem 13:1406–1431. https://doi.org/10.2174/138527209789055090
Padwa A, Tomioka Y, Venkatramanan MK (1987) A study of the 5-exo methylene-isoxazolidine to 3-pyrrolidinone rearrangement. Tetrahedron Lett 28:755–758. https://doi.org/10.1016/S0040-4039(01)80981-3
Padwa A, Matzinger M, Tomioka Y, Venkatramanan MK (1988) Study of the thermal transformation of the 5-exo methyleneisoxazolidines to 3-pyrrolidinones. J Org Chem 53:955–963. https://doi.org/10.1021/jo00240a005
Padwa A, Kline DN, Koehler KF, Matzinger M, Venkatramanan MK (1987) Cycloaddition of nitrones with allenes. An example of steric control of regiochemistry. J Org Chem 52:3909–3917. https://doi.org/10.1021/jo00226a035
Padwa A, Carter SP, Chiacchio U, Kline DN (1986) Dipolar cycloaddition reaction of (phenylsulfonyl)propadiene with nitrones and alkylation studies of the cycloadducts. Tetrahedron Lett 27:2683–2686. https://doi.org/10.1016/S0040-4039(00)84616-X
Padwa A, Bullock WH, Kline DN, Perumattam J (1989) Heterocyclic synthesis via the reaction of nitrones and hydroxylamines with substituted allenes. J Org Chem 54:2862–2869. https://doi.org/10.1021/jo00273a018
Dolbier WR Jr, Wicks GE, Burkholder CR (1987) The cycloadditions of nitrones with fluoroallene. J Org Chem 52:2196–2201. https://doi.org/10.1021/jo00387a015
Alkayar ZTI, Coldham I (2019) Cascade cyclization and intramolecular nitrone dipolar cycloaddition and formal synthesis of 19-hydroxyibogamine. Org Biomol Chem 17:66–73. https://doi.org/10.1039/C8OB02839G
Lee W, Yuan M, Acha C, Onwu A, Gutierrez O (2019) Mechanism of nitrones and allenoates cascade reactions for the synthesis of dihydro[1,2-a] indoles. Org Biomol Chem 17:1767–1772. https://doi.org/10.1039/C8OB02346H
LeBel NA, Banucci E (1970) Intramolecular nitrone-allene cycloadditions. J Am Chem Soc 92:5278–5280. https://doi.org/10.1021/ja00720a080
Padwa A, Meske M, Ni Z (1993) Intramolecular [3+2]-cycloaddition of nitrones with allenes and alkynes. Tetrahedron Lett 34:5047–5050. https://doi.org/10.1016/S0040-4039(00)60672-X
Domingo LR (2016) Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules 21:1319. https://doi.org/10.3390/molecules21101319
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur J Org Chem 267–282 https://doi.org/10.1002/ejoc.201800916
Domingo LR, Acharjee N (2020) In: Ul-Haq Z, Wilson AK (ed) Molecular electron density theory: a new theoretical outlook on organic chemistry. Front Comput Chem 5:174–227. https://doi.org/10.2174/9789811457791120050007
Domingo LR, Acharjee N (2020) Unravelling the strain-promoted [3+2] cycloaddition reactions of phenyl azide with cycloalkynes from the molecular electron density theory perspective. New J Chem 44:13633–13643. https://doi.org/10.1039/D0NJ02711A
Domingo LR, Acharjee N (2020) Unveiling the high reactivity of strained dibenzocyclooctyne in [3+2] cycloaddition reactions with diazoalkanes through the molecular electron density theory. J Phys Org Chem 33:e4100. https://doi.org/10.1002/poc.4100
Domingo LR, Ríos-Gutiérrez M, Silvi B, Pérez P (2018) The mysticism of pericyclic reactions: a contemporary rationalisation of organic reactivity based on electron density analysis. Eur J Org Chem 1107–1120 https://doi.org/10.1002/ejoc.201701350
Domingo LR, Acharjee N, Mohammad Salim HA (2020) Understanding the reactivity of trimethylsilyldiazoalkanes participatingin [3+2]cycloaddition reactions towards diethylfumarate with a molecular electron density theory perspective. Organics 1:3–18. https://doi.org/10.3390/org1010002
Domingo LR, Ríos-Gutiérrez M, Acharjee N (2022) A molecular electron density theory study of the Lewis acid catalyzed [3+2] cycloaddition reactions of nitrones with nucleophilic ethylenes. Eur J Org Chem e202101417 https://doi.org/10.1002/ejoc.202101417
Domingo LR, Ríos-Gutiérrez M, Pérez P (2018) A molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3 + 2] cycloaddition reactions. J Org Chem 83:10959–10973. https://doi.org/10.1021/acs.joc.8b01605
Domingo LR, Acharjee N (2021) Unveiling the substituent effects in the stereochemistry of [3+2] cycloaddition reactions of aryl- and alkyldiazomethylphosphonates with norbornadiene within a MEDT perspective. ChemistrySelect 6:10722–10733. https://doi.org/10.1002/slct.202102942
Domingo LR, Acharjee N (2018) [3+2] Cycloaddition reaction of C-phenyl-N- methyl nitrone to acyclic-olefin-bearing-electron-donating substituent: a molecular electron density theory study. ChemistrySelect 3:8373–8380. https://doi.org/10.1002/slct.201801528
Domingo LR, Ríos-Gutiérrez M, Pérez P (2018) A molecular electron density theory study of the reactivity and selectivities in [3 + 2] cycloaddition reactions of C, N-dialkyl nitrones with ethylene derivatives. J Org Chem 83:2182–2197. https://doi.org/10.1021/acs.joc.7b03093
Domingo LR, Ríos-Gutiérrez M, Adjieufack AI, Ndassa IM, Nouhou CN, Mbadcam JK (2018) Molecular electron density theory study of fused regioselectivity in the intramolecular [3+2] cycloaddition reaction of nitrones. ChemistrySelect 3:5412–5420. https://doi.org/10.1002/slct.201800224
Acharjee N, Mohammad Salim HA, Chakraborty M, Rao MP, Ganesh M (2021) Unveiling the high regioselectivity and stereoselectivity within the synthesis of spirooxindolenitropyrrolidine: a molecular electron density theory perspective. J Phys Org Chem 34:e4189. https://doi.org/10.1002/poc.4189
Acharjee N (2020) Unravelling the regio- and stereoselective synthesis of bicyclic N, O- nucleoside analogues within the molecular electron density theory perspective. Struct Chem (Springer) 31:2147–2160
Domingo LR, Acharjee N (2021) Unveiling the chemo- and regioselectivity of the [3+2] cycloaddition reaction between 4-chlorobenzonitrile oxide and β-aminocinnamonitrile with a MEDT perspective. ChemistrySelect 6:4521–4532. https://doi.org/10.1002/slct.202100978
Domingo LR, Ríos-Gutiérrez M, Acharjee N (2019) A molecular electron density theory study of the chemoselectivity, regioselectivity, and diastereofacial selectivity in the synthesis of an anticancer spiroisoxazoline derived from α-santonin. Molecules 24:832. https://doi.org/10.3390/molecules24050832
Acharjee N, Mondal A, Chakraborty M (2022) Unveiling the intramolecular [3 + 2] cycloaddition reactions of C, N-disubstituted nitrones from the molecular electron density theory perspective. New J Chem 46:7721–7733. https://doi.org/10.1039/d2nj00888b
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397. https://doi.org/10.1063/1.458517
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:683–686. https://doi.org/10.1038/371683a0
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748. https://doi.org/10.3390/molecules21060748
Geerlings P, Proft FD, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874. https://doi.org/10.1021/cr990029p
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428. https://doi.org/10.1039/C4RA04280H
Krokidis X, Noury S, Silvi B (1997) Characterization of elementary chemical processes by catastrophe theory. J Phys Chem A 101:7277–7282. https://doi.org/10.1021/jp9711508
Bader RFW (1994) Atoms in molecules: a quantum theory. Oxford University Press, Oxford, New York
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943. https://doi.org/10.1063/1.446956
García JC, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Hehre WJ, Radom L, PvR S, Pople J (1986) In: AB INITIO molecular orbital theory. Wiley-Interscience, New York
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Cancès E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Barone V, Cossi M, Tomasi J (1998) Monte-Carlo model for the hydrogenation of alkenes on metal catalyst. J Comput Chem 19:404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4%3c404::AID-JCC3%3e3.0.CO;2-W
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163. https://doi.org/10.1021/j100717a029
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
González C, Schlegel HB (1991) Improved algorithms for reaction path following: Higher-order implicit algorithms. J Chem Phys 95:5853–5860. https://doi.org/10.1063/1.461606
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Parr RG, Yang W (1989) In: Density-functional theory of atoms and molecules. Oxford University Press, New York
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9:7168–7175. https://doi.org/10.1039/C1OB05856H
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision D.01. Gaussian, Inc., Wallingford CT
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Molec Graphics 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Domingo LR, Chamorro E, Perez P (2010) Understanding the high reactivity of the azomethine ylides in [3+2] cycloaddition reactions. Lett Org Chem 7:432–439. https://doi.org/10.2174/157017810791824900
Domingo LR, Ríos-Gutiérrez M (2017) A molecular electron density theory study of the reactivity of azomethine imine in [3+2] cycloaddition reactions. Molecules 22:750. https://doi.org/10.3390/molecules22050750
Ríos-Gutiérrez M, Domingo LR (2019) The carbenoid-type reactivity of simplest nitrile imine from a molecular electron density theory perspective. Tetrahedron 75:1961–1967. https://doi.org/10.1016/j.tet.2019.02.014
Domingo LR, Ríos-Gutiérrez M, Perez P (2020) A molecular electron density theory study of the participation of tetrazines in aza-Diels–Alder reactions. RSC Adv 10:15694–15405. https://doi.org/10.1039/D0RA01548B
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Barsali Banerjee under the supervision of Nivedita Acharjee. The draft of the manuscript was written and subsequently reviewed by Barsali Banerjee, Nivedita Acharjee and Debnath Palit. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, B., Acharjee, N. & Palit, D. Revealing the cyclization selectivity in intramolecular [3 + 2] cycloaddition reactions of allenic nitrones from the molecular electron density theory perspective. Struct Chem 35, 209–221 (2024). https://doi.org/10.1007/s11224-023-02175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02175-3