Abstract
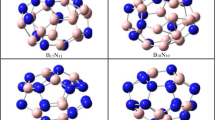
Using the DFT methods, we computationally predict the stability of cage compounds E4nRn (E = B, C; R = H, F; n = 4, 8, 12, 24) based on Platonic bodies and Archimedean polyhedrons in which all vertices are replaced by tetrahedral E4R fragments. Cage compounds B60R12 and C60 with pyramidal units B5R or C5 are also examined and it is shown that only boron compounds are stable. The nature of chemical bonding in the discussed compounds is analyzed using the AdNDP and NBO methods. The hydrocarbons have classical 2c-2e C-C σ-bonds, while the boron compounds are formed by the polyhedral units with the delocalized multicenter bonds which connected three and more boron atoms. The new example of spherical aromaticity according to the 2(N+1)2 rule in the case of B16F4 with multicenter 16c-2e bonds are revealed. Stable compound B60H12 contains 12 5c-2e B-B bonds.










Similar content being viewed by others
References
Burdett JK, Lee S (1985) Moments method and elemental structures. J Am Chem Soc 107:3063–3082
Johnston RL, Hoffmann R (1989) Superdense carbon, C8: supercubane or analog of .gamma.-silicon? J Am Chem Soc 111:810–819
Cremer D, Kraka E, Joo H, Stearns JA, Zwier TS (2006) Exploration of the potential energy surface of C4H4 for rearrangement and decomposition reactions of vinylacetylene: a computational study. Part I Phys Chem Chem Phys 8:5304–5316
Nemirowski A, Reisenauer HP, Schreiner PR (2006) Tetrahedrane–dossier of an unknown. Chem Eur J 12:7411–7420
Haunschild R, Frenking G (2009) Tetrahedranes. A theoretical study of singlet E4H4 molecules (E = C–Pb and B–Tl). Mol Phys 107:911–922 (and references cited therein)
Maier G, Pfriem S (1978) Tetra-tert-butylcyclopentadienone. Angew Chem Int Ed Engl 17:520–521
Maier G, Neudert J, Wolf O, Pappusch D, Sekiguchi A, Tanaka M, Matsuo T (2002) Tetrakis(trimethylsilyl)tetrahedrane. J Am Chem Soc 124:13819–13826
Minyaev RM, Grabanova TN, Minkin VI (2013) Structural stability of supertetrahedral [n] prismanes and their boron analogues: a quantum-chemical study. Dokl Chem 453:270–272
Minyaev RM, Minkin VI (2013) Supertetrahedral cubane C32H8 and supertetrahedral dodecahedrane C80H20 with tetrahedral C4H fragments in the vertices. Mendeleev Commun 23:131–132
Minyaev RM, Popov IA, Koval VV, Boldyrev AI, Minkin VI (2015) Supertetrahedral B80H20, C80H20, and Al80H20 analogs of dodecahedrane and their substituted molecules. Srtuct Chem 26:223–229
Minyaev RM, Starikov AG, Minkin VI (2016) Supermolecular design: from molecules to solid states. Int J Quant Chem 116:259–264
Ortiz YP, Klein DJ, Liebman JF (2018) Paradigms and paradoxes. Tetrahedral units: dodecahedral super-structures. Srtuct Chem 29:89–96
Olson JK, Boldyrev AI (2011) Ab initio search for global minimum structures of neutral and anionic B4H4 clusters. Chem Phys 379:1–5
Mennekes T, Paetzold P, Boese R, Bläser D (1991) Tetra-tert-butyltetraboratetrahedrane. Angew Chem Int Ed Engl 30:173–175
Neu A, Mennekes T, Paetzold P, Englert U, Hofmann M, Schleyer PR (1999) Novel tetraalkyltetraboranes of the type B4R4, B4H2R4 and B4H4R4. Inorg Chim Acta 289:58–69
Ahmed L, Castillo J, Morrison JA (1992) Chemistry of tetraboron tetrachloride. Synthesis and characterization of tetraboron tetrabromide (B4Br4) and observation of B4BrCl3, B4Br2Cl2, and B4Br3Cl. Inorg Chem 31:1858–1860 (and references cited therein)
See full list of references in Ref. 15
Lewars E (1998) Pyramidane: an ab initio study of the C5H4 potential energy surface. J Mol Struct (THEOCHEM) 423:173–188
Lewars E (2000) Pyramidane 2. Further computational studies: potential energy surface, basicity and acidity, electron-withdrawing and electron-donating power, ionization energy and electron affinity, heat of formation and strain energy, and NMR chemical shifts. J Mol Struct (THEOCHEM) 507:165–184
Kenny JP, Krueger KM, Rienstra-Kiracofe JC, Schaefer III HF (2001) C5H4: Pyramidane and its low-lying isomers. J Phys Chem A 105:7745–7750
Minkin VV, Minyaev RM (2002) Pyramidane and pyramidal cations. Dokl Chem 385:203–206
McKee ML (1999) Ab initio study of BnHn and Bn(NH2)n (n=3–6) species. A comparison of classical and nonclassical structures. Inorg Chem 38:321–330
McKee ML, Wang Z-X, Schleyer PR (2000) Ab initio study of the hypercloso boron hydrides BnHn and BnHn −. Exceptional stability of neutral B13H13. J Am Chem Soc 122:4781–4793
Lee VY, Ito Y, Sekiguchi A, Gornitzka H, Gapurenko OA, Minkin VI, Minyaev RM (2013) Pyramidanes. J Am Chem Soc 135:8794–8797
Lee VY, Gapurenko OA, Ito Y, Meguro T, Sugasawa H, Sekiguchi A, Minyaev RM, Minkin VI, Herber RH, Gornitzka H (2016) Pyramidanes: the covalent form of the ionic compounds. Organometallics 35:346–356
Lee VY, Sugasawa H, Gapurenko OA, Minyaev RM, Minkin VI, Gornitzka H, Sekiguchi A (2018) From borapyramidane to borole dianion. J Am Chem Soc 140:6053–6056
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari R, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09. Revision E.01. Gaussian Inc, Wallingford CT
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) NBO 6.0. Theoretical Chemistry Institute. University of Wisconsin. Madison
Zubarev DY, Boldyrev AI (2008) Develo** paradigms of chemical bonding: adaptive natural density partitioning. Phys Chem Chem Phys 10:5207–5217
Zhurko GA.ChemCraft software. http://www.chemcraftprog.com. Accessed 1 May 2019
Schleyer PR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Pyykkö P, Atsumi M (2009) Molecular double-bond covalent radii for elements Li–E112. Chem Eur J 15:12770–12779
Hirsch A, Chen Z, Jiao H (2000) Spherical aromaticity in I h symmetrical fullerenes: the 2(N+1)2 rule. Angew Chem Int Ed Engl 39:3915–3917
Acknowledgements
The work was supported by the Russian Science Foundation (grant 16-13-10050).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 999 kb)
Rights and permissions
About this article
Cite this article
Gapurenko, O.A., Minyaev, R.M., Fedik, N.S. et al. Structure and bonding of new boron and carbon superpolyhedra. Struct Chem 30, 805–814 (2019). https://doi.org/10.1007/s11224-019-1279-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-1279-5