Abstract
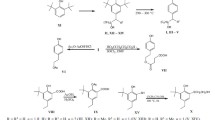
A new method for the synthesis of p-tyrosol, i.e., 2-(4-hydroxyphenyl)ethanol, has been developed, which considerably simplified the process of preparation of this pharmaceutical substance. The method includes nitration of 2-phenylethanol, catalytic hydrogenation of 2-(4nitrophenyl)ethyl nitrate, and nitrosation of 2-(4-aminophenyl)ethanol, followed by acid hydrolysis of the intermediate diazo compound. The method is easily feasible, furnishes the pharmaceutical substance of high quality, and is quite acceptable for commercialization. Antihypertensive and hemorheologic activities of p-tyrosol in spontaneously hypertensive rats were investigated per se and in combination with captopril. p-Tyrosol in the course administration with captopril was found to enhance the antihypertensive effect of the latter and eliminate its negative effect on red blood cell aggregation.
Similar content being viewed by others
References
A. S. Saratikov, E. A. Krasnov, Rodiola rozovaya (zolotoy koreń) [Rhodiola Rosea (Golden Root)], 4th revised and expanded ed., Tomsk Univ. Publ., Tomsk, 2004, 292 pp. (in Russian).
R. Di Benedetto, R. Varì, B. Scazzocchio, C. Filesi, C. Santangelo, C. Giovannini, P. Matarrese, M. D’Archivio, R. Masella, Nutr. Metab. Cardiovasc. Dis., 2007, 17, 535.
H. Chimi, I. Morel, G. Lescoat, N. Pasdeloup, P. Cillard, J. Cillard, Toxicol. In Vitro, 1995, 9, 695.
N. M. Storozhok, N. M. Gureeva, R. A. Khalitova, A. S. Storozhok, A. P. Krysin, Pharm. Chem. J. (Engl. Transl.), 2011, 45, 732 [Khim. Farm. Zh., 2012, 45, No. 12, 23].
C. J. Ma, Y. C. Kim, S. H. Sung, J. Enzyme Inhib. Med. Chem., 2009, 24, 1117.
D. Vasour, G. Corona, J. P. Spencer, Arch. Biochem. Biophys., 2010, 501, 106.
P. Dewapriya, S. W. Himaya, Y. X. Li, S. K. Kim, Food Chem., 2013, 141, 1147.
M. B. Plrelikov, G. A. Chernysheva, V. I. Smolýakova, M. Yu. Oilv, I. V. Cherkashina, A. P. Krysin, I. V. Sorokina, T. G. Tolstikova, Bull. Exp. Biol. Med. (Engl. Transl.), 2007, 143, 61 [Byul. Eksperim. Biol. Med., 2007, 143, 67].
V. I. Smolýakova, G. A. Chernysheva, M. B. Plotnikov, O. I. Aliev, E. A. Krasnov, Kardiologiya [Cardiology], 2010, 50, 47 (in Russian).
G. A. Chernysheva, M. B. Plotnikov, V. I. Smolýakova, I. V. Golubeva, O. I. Aliev, T. G. Tolstikova, A. P. Krysin, I. V. Sorokina, Bull. Exp. Biol. Med. (Engl. Transl.), 2007, 143, 689 [Byul. Eksperim. Biol. Med., 2007, 143, 631].
A. P. Krysin, V. S. Kobrin, I. V. Sorokina, Chem. Sustainable Dev. (Engl. Transl.), 2010, 18, 461 [Khim. v Interesakh Ustoichivogo Razvitiya, 2010, 18, 543]; http://siberian.ru/en/ journals/KhUR.
A.s. 662101 SSSR; Byul. isobret. [Invention Bull.], 1979, No. 18 (in Russian).
A. G. Troshenko, G. A. Kutikova, Chem. Nat. Compd. (Eng. Transl.), 1967, 3, 204 [Khim. Prirod. Soedin., 1967, 3, 244].
Pat. RF 2151137; Byul. isobret. [Invention Bull.], 2000, No. 17 (in Russian).
Pat. EP 0307106; http://worldwide.espacenet.com.
Pat. RF 2385858; Byul. isobret. [Invention Bull.], 2010, No. 10; http://www1.fips.ru (in Russian).
Pat. RF 2063395; Byul. isobret. [Invention Bull.], 1996, No. 19 (in Russian).
A. P. Krysin, T. G. Egorova, V. G. Vasiliev, Russ. J. Gen. Chem. (Eng. Transl.), 2010, 80, 275 [Zh. Obshch. Khim., 2010, 80, 250].
E. Ferber, Chem. Ber., 1929, 62, 183.
H. M. Woodburn, C. F. Stuntz, J. Am. Chem. Soc., 1950, 72, 1361.
V. G. Sinyavskii, V. Ph. Kovaleva, J. Org. Chem. USSA (Eng. Transl.), 1970, 6, 1697 [Zh. Org. Khim., 1970, 6, 1692].
K. K. Muradov, Yu. A. Kryukov, S. V. Sysolyatin, Polzunovskii vestnik [Polzunov Bull.], 2014, 3, 126 (in Russian).
S. Shin, Y. Yang, J.-S. Suh, Korea-Australia Rheol. J., 2007, 19, 109.
S. Shin, Y. Yang, J.-S. Suh, Korea-Australia Rheol. J., 2008, 20, 253.
S. Shin, Y. Ku, M.-S. Park, J.-S. Suh, Korea-Australia Rheol. J., 2004, 16, 85.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to Academician of the Russian Academy of Sciences N. S. Zefirov on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 2210–2214, September, 2015.
Rights and permissions
About this article
Cite this article
Sysolyatin, S.V., Kryukov, Y.A., Malykhin, V.V. et al. p-Tyrosol: a new synthetic method and new types of pharmacological activity. Russ Chem Bull 64, 2210–2214 (2015). https://doi.org/10.1007/s11172-015-1140-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-1140-y