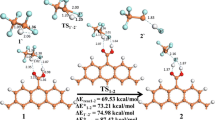
Abstract
In this paper, the conversion of oxygen atoms in Fischer–Tropsch synthesis of low carbon olefin (FTO) catalyzed by Co2C was investigated. Density functional theory (DFT) was used to investigate the surface reaction mechanism. The mechanism of H2O and CO2 formation was studied. Then the rate control steps for the generation of both were found. Research shows the key step in the formation of water is the hydrogenation of O to OH with an activation energy of 1.18 eV. The formation of COOH is the key step to the formation of CO2 with an activation energy of 1.78 eV. It is clear that the formation of H2O is kinetically dominant over that of CO2. The formation frequency of water and CO2 at different temperatures was investigated using the kinetic Monte Carlo (KMC) method, where the conversion frequency of H2O is about 1.6 times higher than that of CO2. The article indicates that O atoms are more readily converted to H2O than CO2 in the FTO process occurring over the Co2C catalyst.
Graphical abstract










Similar content being viewed by others
Data availability
Materials described in the manuscript includes all relevant raw data and are available to any researcher who read this manuscript.
References
Amghizar I, Vandewalle LA, Van Geem KM, Marin GB (2017) New trends in olefin production. Engineering 3(2):171–178. https://doi.org/10.1016/J.ENG.2017.02.006
Tong XG, Zhang GY, Wang ZM, Wen ZX, Tian ZJ, Wang HJ, Ma F, Wu YP (2018) Distribution and potential of global oil and gas resources. Pet Explor Dev 45(4):779–789. https://doi.org/10.1016/S1876-3804(18)30081-8
Fischer FATH (1926) The synthesis of petroleum at atmospheric pressures from gasification products of coal. Brennstoff-Chemie 7:97–104
Yu F, Lin TJ, An YL, Gong K, Wang XX, Sun YH, Zhong LS (2022) Recent advances in Co2C-based nanocatalysts for direct production of olefins from syngas conversion. Chem Commun 58(70):9712–9727. https://doi.org/10.1039/d2cc03048a
Torres Galvis HM, de Jong KP (2013) Catalysts for production of lower olefins from synthesisgas: a review. ACS Catal 9(3):2130–2149
Zhong LS, Yu F, An YL, Zhao YH, Sun YH, Li ZJ, Lin TJ, Lin YJ, Qi XZ, Dai YY, Gu L, Hu JS, ** SF, Shen Q, Wang H (2016) Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 538(7623):84. https://doi.org/10.1038/nature19786
Li ZJ, Yu DM, Yang LY, Cen J, **ao K, Yao N, Li XN (2021) Formation mechanism of the Co2C nanoprisms studied with the CoCe system in the Fischer-Tropsch to Olefin reaction. ACS Catal 11(5):2746–2753. https://doi.org/10.1021/acscatal.0c04504
Zhai P, Li YW, Wang M, Liu JJ, Cao Z, Zhang J, Xu Y, Liu XW, Li YW, Zhu QJ, **ao DQ, Wen XD, Ma D (2021) Development of direct conversion of syngas to unsaturated hydrocarbons based on Fischer–Tropsch route. Chem 7(11):3027–3051. https://doi.org/10.1016/j.chempr.2021.08.019
Zaffran J, Yang B (2021) Theoretical insights into the formation mechanism of methane, ethylene and methanol in Fischer-Tropsch synthesis at Co2C surfaces. ChemCatChem 13(11):2674–2682. https://doi.org/10.1002/cctc.202100216
Chen PPLJXL (2019) Carbon monoxide activation on cobalt carbide for Fischer-Tropsch synthesis from first-principles theory. ACS Catal 9(9):8093–8103
Sahrin NT, Khoo KS, Lim JW, Shamsuddin R, Ardo FM, Rawindran H, Hassan M, Kiatkittipong W, Abdelfattah EA, Da Oh W, Cheng CK (2022) Current perspectives, future challenges and key technologies of biohydrogen production for building a carbon-neutral future: A review. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.128088
Kresse GHJ (1993) Ab initio molecular dynamics for open-shell transition metals. Phys Rev B 17(48):13115
Kresse GFJ (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 16(54):11169
Maintz S, Deringer VL, Tchougreeff AL, Dronskowski R (2013) Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J Comput Chem 34(29):2557–2567. https://doi.org/10.1002/jcc.23424
Henkelman G, Jonsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys 113(22):9978–9985. https://doi.org/10.1063/1.1323224
Henkelman G, Jonsson H (1999) A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys 111(15):7010–7022. https://doi.org/10.1063/1.480097
Metropolis NUS (1949) The monte carlo method. J Am Stat Assoc 247(44):335–341
Voter AF (2007) Introduction to the kinetic monte carlo method. In: Sickafus KE, Kotomin EA, Uberuaga BP (eds) Radiation effects in solids. Springer Netherlands, Dordrecht, pp 1–23
Tian L, Huo CF, Cao DB, Yang Y, Xu J, Wu BS, **ang HW, Xu YY, Li YW (2010) Effects of reaction conditions on iron-catalyzed Fischer-Tropsch synthesis: A kinetic Monte Carlo study. J Mol Struct-Theochem 941(1–3):30–35. https://doi.org/10.1016/j.theochem.2009.10.032
Lin S, Ma JY, Ye XX, **e DQ, Guo H (2013) CO Hydrogenation on Pd(111): competition between Fischer-Tropsch and oxygenate synthesis pathways. J Phys Chem C 117(28):14667–14676. https://doi.org/10.1021/jp404509v
Jansen A, Agrawal R, Spanu L (2016) Thermodynamics and kinetics of carbon deposits on cobalt: a combined density functional theory and kinetic Monte Carlo study. Phys Chem Chem Phys 18(41):28515–28523. https://doi.org/10.1039/c6cp04719j
Amaya-Roncancio S, Arroyo-Gomez JJ, Linares DH, Sapag K (2020) Direct versus hydrogen-assisted dissociation of CO on iron surfaces: Kinetic Monte Carlo and microkinetic modeling. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.127188
Huang HY, Yu YZ, Zhang MH (2020) CO dissociation mechanism on Mn-Doped Fe(100) Surface: a computational investigation. Catal Lett 150(6):1618–1627. https://doi.org/10.1007/s10562-019-03066-1
Yu YZ, Zhang J, Lei H, Zhang MH (2020) Carbon chain growth reaction of synthesis of lower olefins from syngas on Fe–Co catalyst. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2019.144211
Zhang MH, Chi SC, Huang HY, Yu YZ (2021) Mechanism insight into MnO for CO activation and O removal processes on Co(0001) surface: A DFT and kMC study. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2021.150854
Zhang MH, Yu HP, Yu YZ, Wang LT (2022) Key roles of formyl insertion mechanism and C-O scission of oxygenates on cobalt carbide in syngas Conversion: a detailed reaction network analysis. J Catal 413:455–466. https://doi.org/10.1016/j.jcat.2022.07.005
Zhang MH, Yu HP, Sun YZ, Yu YZ, Chen YF, Wang LT (2022) Theoretical and experimental insights into CO2 formation on Co2C catalysts in syngas conversion to value-added chemicals. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2022.154379
Manuel O, Rahul N, Anand UN, Akio I (2010) CO activation pathways and the mechanism of Fischer–Tropsch synthesis. J Catal 272(2):287–297
Henkelman G (2017) Atomistic simulations of activated processes in materials. Ann Rev Mater Res. https://doi.org/10.1146/annurev-matsci-071312-121616
Karim W, Spreafico C, Kleibert A, Gobrecht J, VandeVondele J, Ekinci Y, van Bokhoven JA (2017) Catalyst support effects on hydrogen spillover. Nature 541(7635):68. https://doi.org/10.1038/nature20782
Huai L-y STWH (2016) NO reduction by H2 on the Rh(111) and Rh(221) Surfaces: a mechanistic and kinetic study. J Phys Chem C 10(120):5410–5419
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 21978199)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Geng, Z., **ao, C., Gao, J. et al. Theoretical research of the main conversion path of oxygen atom on Co2C catalysts in the Fischer–Tropsch synthesis process. Reac Kinet Mech Cat 136, 1915–1932 (2023). https://doi.org/10.1007/s11144-023-02436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02436-6