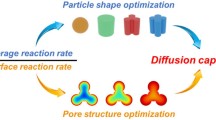
Abstract
In this paper, different geometries and types of catalyst distribution used for fixed bed WGS reactors that can be placed at the outlet of gasifiers have been studied. It has been shown that spherical eggshell catalysts allow significant reductions in catalytic mass compared to uniform pellets. Moreover, thanks to the possibility of using large pellets offered by eggshell catalysts, the pressure drops observed when using these particles remain acceptable (< 5%) even for a long reactor (Z = 2.8 m) and despite a low feed pressure (P0 = 2.5 atm). Simulations also revealed that operating in isothermal or isoperibolic mode at low temperature (T = 630 K) improves significantly the process efficiency.









Similar content being viewed by others
Abbreviations
- Ap :
-
Surface area of catalyst exposed to surrounding fluid (m2)
- CCO,Eq :
-
O concentration at chemical equilibrium (mol m−3)
- Ci :
-
Concentration of species i (mol m−3)
- Ci,S :
-
Concentration of species i at the particle surface (mol m−3)
- Ci,F :
-
Concentration of species i in the bulk phase (mol m−3)
- Cpi :
-
Heat capacity at constant pressure of species i (J mol−1 K−1)
- Cpw :
-
Specific heat capacity of coolant (J kg−1 K−1)
- dp :
-
Equivalent particle diameter (m)
- dpore :
-
Average pore size (m)
- DCO,Ax :
-
Axial dispersion coefficient of CO (m2 s−1)
- De ,I :
-
Effective diffusion coefficient of species i (m2 s−1)
- Dext :
-
External Diameter of inner pipe (m)
- Dia :
-
Internal diameter of outer pipe (m)
- Dt :
-
Internal Diameter of inner pipe (m)
- e :
-
Thickness of slab (m)
- eactive :
-
Thickness of the active phase layer (m)
- et :
-
Thickness of reactor tube (m)
- Fi:
-
Molar flow rate of species i (mol s−1)
- Fi,0 :
-
Feed stream of species i (mol s−1)
- Fpress :
-
Pressure scale-up factor
- h:
-
Heat-transfer coefficient (J s−1 m−2 K−1)
- hi :
-
Individual heat transfer coefficient for packed bed reactor side (J s−1 m−2 K−1)
- h0 :
-
Individual heat transfer coefficient at the annulus side of the reactor (J s−1 m−2 K−1)
- KC,I :
-
Mass-transfer coefficient of species i (m s−1)
- Ke :
-
Equilibrium constant of the WGS reaction
- ls :
-
Width of slab (m)
- L:
-
Length of reactor (m)
- Lc :
-
Length of cylindrical particle (m)
- Ls :
-
Length of slab (m)
- mw :
-
Mass flow rate of coolant (kg s−1)
- Nt :
-
Number of tubes
- P:
-
Pressure (Pa)
- Pr:
-
Prandlt number
- r:
-
Intraparticle spatial position (m)
- rCOf :
-
Reaction rate under bulk conditions (mol kg−1 s−1)
- rCO(r) :
-
Reaction rate at r position (mol kg−1 s−1)
- rCOs :
-
Reaction rate under surface conditions (mol kg−1 s−1)
- rCO(tactive):
-
Reaction rate at tactive position (mol kg−1 s−1)
- \(\overline{{r }_{CO}}\) :
-
Apparent reaction rate (mol kg−1 s−1)
- R:
-
Radius of the particle (m)
- Rg :
-
Universal gas constant (8.3144 J K−1 mol−1)
- Rinerte :
-
Radius of the inert core (m)
- Re:
-
Reynolds’ number
- S:
-
Cross section of reactor tube (m2)
- Sc:
-
Schmidt number of CO
- tactive :
-
Spatial position inside the active phase layer (m)
- T:
-
Temperature (K)
- TC :
-
Temperature of coolant (K)
- Tf :
-
Temperature of the bulk gas (K)
- Ts:
-
Temperature of the surface of the pellet (K)
- us :
-
Superficial velocity (m s−1)
- U:
-
Overall heat transfer coefficient between the jacket and the reactor (W m−2 K−1)
- Vp :
-
Particle volume (m3)
- XCO :
-
Carbon monoxide conversion
- z:
-
Reactor axial coordinate (m)
- ∆HR :
-
Enthalpy of the WGS reaction (J mol−1)
- ∆Tmax :
-
Maximum temperature variation between the center and the surface of the pellet (K)
- εb :
-
Porosity of catalytic bed
- εC :
-
Intragranular porosity
- ɳe :
-
Overall effectiveness factor
- ɳeactive :
-
Overall effectiveness factor of the active phase layer
- ɳs :
-
Effectiveness factor of the particle
- λe :
-
Effective thermal conductivity (J m−1 s−1 K−1)
- λg :
-
Thermal conductivity of gas mixture (J m−1 s−1 K−1)
- λS :
-
Thermal conductivity of solid (J m−1 s−1 K−1)
- λt :
-
Thermal conductivity of tube wall (J m−1 s−1 K−1)
- λw :
-
Thermal conductivity of coolant (J m−1 s−1 K−1)
- µ:
-
Dynamic viscosity of gas-mixture (kg m−1 s−1)
- µw :
-
Dynamic viscosity of coolant (kg m−1 s−1)
- ρ:
-
Density of gas-mixture (kg m−3)
- ρB :
-
Density of bed (kg m−3)
- ρc :
-
Density of catalyst (kg m−3)
- τ:
-
Pore tortuosity
- \({\Phi }_{s}\) :
-
Thiele modulus
- GHSV:
-
Gas hourly space velocity (h−1)
- RK4:
-
Runge–Kutta 4th Order
- WGS:
-
Water Gas Shift
References
Chi J, Hongmei Yu (2018) Water electrolysis based on renewable energy for hydrogen production. Chin J Catal 39:390–394. https://doi.org/10.1016/S1872-2067(17)62949-8
Menia S, Nouicer I, Bakouri Y, M’raoui A, Tebibel H, Khellaf A (2019) Production d’hydrogène par procédes biologiques. Oil Gas Sci Technol 74:1–16. https://doi.org/10.2516/ogst/2018099
El Bazi W, Bideq M, El Abidi A, Yadir S, Ouartassi B (2022) Numerical study of a water gas shift fixed bed reactor operating at low pressures. Bull Chem Reaction Eng Catal 17:304–321. https://doi.org/10.9767/bcrec.17.2.13510.304-321
Lang C (2016) Développement de catalyseurs pour la réaction de conversion du gaz à l’eau dans le cadre de la production d’hydrogène par vapogazéification de la biomasse. PhD thesis (in French). Strasbourg : Strasbourg University, France
Lang C, Sécordel X, Kiennemann A, Courson C (2017) Water gas shift catalysts for hydrogen production from biomass steam gasification. Fuel Process Technol 156:246–252. https://doi.org/10.1016/j.fuproc.2016.09.004
Lang C, Secordel X, Courson C (2017) Copper-based water gas shift catalysts for hydrogen rich syngas production from biomass steam gasification. Energy Fuels 31:12932–12941. https://doi.org/10.1021/acs.energyfuels.7b01765
Keiski RL, Salmi T, Pohjola VJ (1992) Development and verification of a simulation model for a non-isothermal water gas-shift reactor. Chem Eng J 48:17–29. https://doi.org/10.1016/0300-9467(92)85003-R
Keiski RL, Desponds O, Chang YF, Somorjai GA (1993) Kinetics of the water-gas shift reaction over sevral alkane activation and water–gas shift catalyst. Appl Catal A 101:317–338. https://doi.org/10.1016/0926-860X(93)80277-W
Koryabkina NA, Phatak AA, Ruettinger WF, Farrauto RJ, Ribeiro FH (2003) Determination of kinetic parameters for the water-gas-shift reaction on copper catalysts under realistic conditions for fuel cell applications. J Catal 217:233–239. https://doi.org/10.1016/S0021-9517(03)00050-2
Hla SS, Park D, Duffy GJ, Edwards JH, Roberts DG (2009) Kinetics of high-temperature water-gas shift reaction over two iron-based commercial catalysts using simulated coal-derived syngases. Chem Eng J 146:148–154. https://doi.org/10.1016/j.cej.2008.09.023
Atwood K, Arnold MR, Appel EG (1950) Water–gas shift reaction. Effect of pressure on rate over an iron–oxide–chromium oxide catalyst. Ind Eng Chem 42:1600–1602. https://doi.org/10.1021/ie50488a038
Pengmei L, Chuangzhi W, Longlong M, Zhenhong Y (2008) A study on the economic efficiency of hydrogen production from biomass residues in China. Renewable Energy 33:1874–1879. https://doi.org/10.1016/j.renene.2007.11.002
Darmawan A, Aziz M (2021) Innovative energy conversion from biomass waste. Elsevier, Amsterdam
Moneti M, Di Carlo A, Bocci E, Foscolo PU, Villarini M, Carlini M (2016) Influence of the main gasifier parameters on a real system for hydrogen production from biomass. Int J Hydrogen Energy 41:11965–11973. https://doi.org/10.1016/j.ijhydene.2016.05.171
Hallac BB (2014) Kinetic experimental and modeling studies on iron-based catalysts promoted with lanthana for the high-temperature water-gas shift reaction characterized with operando UV–Visible spectroscopy and for the Fischer-Tropsch synthesis. PhD thesis. Provo: Brigham Young University, USA
El Bazi W, El Abidi A, Kadiri MS, Yadir S (2018) Modeling and simulation of a water gas shift reactor operating at a low pressure. Int J Innov Eng Sci Res 2:47–57
Gancarczyk A, Sindera K, Iwaniszyn M, Piatek M, Macek W, Jodłowski P, Wronski S, Sitarz M, Łojewska J, Kołodziej A (2019) Metal foams as novel catalyst support in environmental processes. Catalysts 9(587):1–13. https://doi.org/10.3390/catal9070587
Becker ER, Wei J (1977) Non-uniform distribution of catalysts on supports. II. First order reactions with poisoning. J Catal 46:372–381. https://doi.org/10.1016/0021-9517(77)90221-4
Zimmermann RT, Bremer J, Sundmacher K (2020) Optimal catalyst particle design for flexible fxed-bed CO2 methanation reactors. Chem Eng J 387(123704):1–16. https://doi.org/10.1016/j.cej.2019.123704
Iglesias I, Fraccari EP, Giunta P (2020) Study of efectiveness factors with non-uniform catalyst distributions for methane steam reforming. React Kinet Mech Catal 130:713–726. https://doi.org/10.1007/s11144-020-01822-8
Wang Y-N, Xu Y-Y, **ang H-W, Li Y-W, Zhang B-J (2001) Modeling of catalyst pellets for Fischer–Tropsch synthesis. Ind Eng Chem Res 40:4324–4335. https://doi.org/10.1021/ie010080v
Wang PW, Du Y, Shen WD, Liang JJ, Xu JF (2013) Numerical simulation of catalytic combustion based on different catalyst structures. Adv Mater Res 791–793:311–314. https://doi.org/10.4028/www.scientific.net/AMR.791-793.311
Lim S, Bae J, Kim K (2009) Study of activity and effectiveness factor of noble metal catalysts for water–gas shift reaction. Int J Hydrogen Energy 34:870–876. https://doi.org/10.1016/j.ijhydene.2008.10.048
Iglesia E, Soled SL, Baumgartner JE, Reyes SC (1995) Synthesis and catalytic properties of eggshell cobalt catalysts for the Fischer–Tropsch synthesis. J Catal 153:108–122. https://doi.org/10.1006/jcat.1995.1113
Russo V, Mastroianni L, Tesser R, Salmi T, Di Serio M (2020) Intraparticle modeling of non-uniform active phase distribution catalyst. ChemEngineering 4(24):1–15. https://doi.org/10.3390/chemengineering4020024
Mazidi SK, Sadeghi MT, Marvast MA (2013) Optimization of Fischer–Tropsch process in a fixed-bed reactor using non-uniform catalysts. Chem Eng Technol 36:62–72. https://doi.org/10.1002/ceat.201200268
Mandic M, Branislav T, Zivanic L, Nikacevic N, Bukur DB (2017) Effects of catalyst activity, particle size and shape, and process conditions on catalyst effectiveness and methane selectivity for Fischer–Tropsch reaction: a modeling study. Ind Eng Chem Res 56:2733–2745. https://doi.org/10.1021/acs.iecr.7b00053
Soled SL, Baumgartner JE, Reyes SC, Iglesia E (1995) Synthesis of eggshell cobalt catalysts by molten salt impregnation techniques. Stud Surf Sci Catal 91:989–997. https://doi.org/10.1016/S0167-2991(06)81842-2
Peluso E, Galarraga C, De Lasa H (2001) Eggshell catalyst in Fischer–Tropsch synthesis: intrinsic reaction kinetics. Chem Eng Sci 56:1239–1245. https://doi.org/10.1016/S0009-2509(00)00345-6
Zhu Q, Gao J, Chen J, Wen L-X (2010) Selective hydrogenation of acetylene over egg-shell palladium nano-catalyst. J Nanosci Nanotechnol 10:5641–5647. https://doi.org/10.1166/jnn.2010.2472
Cho E, Yu YJ, Kim Y, Phan TN, Park D, Ko CH (2020) Egg-shell-type Ni supported on MgAl2O4 pellets as catalyst for steam methane reforming: enhanced coke-resistance and pellet stability. Catal Today 352:157–165. https://doi.org/10.1016/j.cattod.2019.11.013
Macias MJ, Ancheyta J (2004) Simulation of an isothermal hydrodesulfurization small reactor with different catalyst particle shapes. Catal Today 98:243–252. https://doi.org/10.1016/j.cattod.2004.07.038
Soltan Mohammadzadeh JS, Zamaniyan A (2002) Catalyst shape as a design parameter—optimum shape for methane-steam reforming catalyst. Chem Eng Res Des 80:383–391. https://doi.org/10.1205/026387602317446425
Guinta P, Amadeo N, Laborde M (2006) Simulation of a low temperature water gas shift reactor using the heterogeneous model/application to a pem fuel cell. J Power Sources 156:489–496. https://doi.org/10.1016/j.jpowsour.2005.04.036
Zhang L, Zhang HT, Ying WY, Fang DY (2014) The simulation of an industrial fixed bed reactor for methanol dehydration to dimethyl ether. Energy Sources A 36:2166–2174. https://doi.org/10.1080/15567036.2012.750404
Tavan Y, Shariati A, Nikou MRK (2015) Validation of experimental results through reactor modeling for catalyzed dehydration of methanol reaction. Energy Sources Part A 37:1210–1218. https://doi.org/10.1080/15567036.2011.605432
E4tech (2009) Review of technology for the gasification of biomass and wastes https://www.e4tech.com/resources/95-review-of-technologies-for-gasification-of-biomass-and-wastes.php?filter=year%3A2009. accessed 19 Oct 2022
Hrbek J (2022) Status report on thermal gasification of biomass and waste 2021. IEA Bioenergy https://www.ieabioenergy.com/wp-content/uploads/2022/03/Status-Report2021_final.pdf. accessed 19 Oct 2022
Elnashaie SSEH, Alhabdan F (1989) Mathematical modelling and computer simulation of industrial Water–Gas shift converters. Math Comput Model 12:1017–1034. https://doi.org/10.1016/0895-7177(89)90208-2
Adams TA, Barton PI (2009) A dynamic two-dimensional heterogeneous model for water gas shift reactors. Int J Hydrogen Energy 34:8877–8891. https://doi.org/10.1016/j.ijhydene.2009.08.045
Van Dijk HAJ, Cohen D, Hakeem AA, Makkee M, Damen K (2014) Validation of a water–gas shift reactor model based on a commercial FeCr catalyst for pre-combustion CO2 capture in an IGCC power plant. Int J Greenhouse Gas Control 29:82–91. https://doi.org/10.1016/j.ijggc.2014.07.005
Rosner F, Rao A, Samuelsen S (2020) Water gas shift reactor modelling and new dimensionless number for thermal management/design of isothermal reactors. Appl Therm Eng 173:1–19. https://doi.org/10.1016/j.applthermaleng.2020.115033
Kuncharam BVR, Dixon AG (2020) Multi-scale two-dimensional packed bed reactor model for industrial steam methane reforming. Fuel Process Technol 200(106314):1–14. https://doi.org/10.1016/j.fuproc.2019.106314
Soto V, Ulloa C, Garcia X (2022) A 3D transient CFD simulation of a multi-tubular reactor for power to gas applications. Energies 15(3383):1–21. https://doi.org/10.3390/en15093383
Zhu J, Cui X, Araya SS (2022) Comparison between 1D and 2D numerical models of a multi-tubular packed-bed reactor for methanol steam reforming. Int J Hydrogen Energy 47:22704–22719. https://doi.org/10.1016/j.ijhydene.2022.05.109
Petric I, Karić E (2019) Simulation of commercial fixed-bed reactor for maleic anhydride synthesis: application of different kinetic models and industrial process data. Reac Kinet Mech Cat 126:1027–1054. https://doi.org/10.1007/s11144-019-01533-9
Suhan MBK, Hemal MNR, Choudhury MAAS, Mazumder MAA, Islam M (2020) Optimal design of ammonia synthesis reactor for a process industry. J King Saud Univ Eng Sci 34:23–30. https://doi.org/10.1016/j.jksues.2020.08.004
Behloul CR, Commenge J-M, Castel C (2021) Simulation of reactors under different thermal regimes and study of the internal diffusional limitation in a fixed-bed reactor for the direct synthesis of dimethyl ether from a CO2-rich input mixture and H2. Ind Eng Chem Res 60:1602–1623. https://doi.org/10.1021/acs.iecr.0c05535
Vernikovskaya N, Ivanova Y, Sheboltasov A, Chumachenko V, Isupova L (2023) Modeling of a two-bed reactor for low-temperature removal of nitrogen oxides in nitric acid production. Catalysts 13(535):1–15. https://doi.org/10.3390/catal13030535
Hwang S, Smith R (2004) Heterogeneous catalytic reactor design with optimum temperature profile I: application of catalyst dilution and side-stream distribution. Chem Eng Sci 59:4229–4243. https://doi.org/10.1016/j.ces.2004.05.037
Vadlamudi VK, Palanki S (2011) Modeling and analysis of miniaturized methanol reformer for fuel cell powered mobile applications. Int J Hydrogen Energy 36:3364–3370. https://doi.org/10.1016/j.ijhydene.2010.12.062
Sanz R, Calles JA, Alique D, Furones L, Ordonez S, Marin P (2015) Hydrogen production in a pore-plated Pd-membrane reactor: experimental analysis and model validation for the Water Gas Shift reaction. Int J Hydrogen Energy 40:3472–3484. https://doi.org/10.1016/j.ijhydene.2014.11.120
Santacesaria E, Tesser R (2018) The chemical reactor from laboratory to industrial plant: a modern approach to chemical reaction engineering with different case histories and exercises. Springer, Cham
Wakao N, Kaguei S, Funazkri T (1979) Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed-beds—Correlation of Nusselt numbers. Chem Eng Sci 34:325–336. https://doi.org/10.1016/0009-2509(79)85064-2
Francesconi JA, Mussati MC, Aguirre PA (2007) Analysis of design variables for water–gas-shift reactors by model-based optimization. J Power Sources 173:467–477. https://doi.org/10.1016/j.jpowsour.2007.04.048
Missen RW, Mims CA, Saville BA (1998) Introduction to chemical reaction engineering and kinetics. Wiley, Danvers
McCabe W, Smith J, Harriot P (2000) Unit operations of chemical engineering, 6th edn. McGraw-Hill, New York
Davis ME, Davis RJ (2003) Fundamentals of chemical reaction engineering. McGraw-Hill, New York
Villermaux J (1993) Génie de la réaction chimique, 2nd edn. Tec & Doc, Paris
Villadsen JV, Stewart WE (1967) Solution of boundary-value problems by orthogonal collocation. Chem Eng Sci 22:1483–1501. https://doi.org/10.1016/0009-2509(67)80074-5
Nodehi A, Mousavian MA (2006) Simulation and optimization of an adiabatic multi-bed catalytic reactor for the oxidation of SO2. Chem Eng Technol 29:84–90. https://doi.org/10.1002/ceat.200600231
Lee WJ, Froment GF (2008) Ethylbenzene dehydrogenation into styrene: kinetic modeling and reactor simulation. Ind Eng Chem Res 47:9183–9194. https://doi.org/10.1021/ie071098u
Forment GF, Bischoff KB, Wilde JD (2010) Chemical reactor analysis and design, 3rd edn. Wiley, Hoboken
Marin P, Diez FV, Ordonez S (2012) Fixed bed membrane reactors for WGSR based hydrogen production: optimisation of modelling approaches and reactor performance. Int J Hydrogen Energy 37:4997–5010. https://doi.org/10.1016/j.ijhydene.2011.12.027
Maklavany DM, Shariati A, Nikou MRK, Roozbehani B (2017) Hydrogen production via low temperature water gas shift reaction: kinetic study, mathematical modeling, simulation and optimization of catalytic fixed bed reactor using gPROMS. Chem Product Process Model. https://doi.org/10.1515/cppm-2016-0063
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Bazi, W., Bideq, M., Yadir, S. et al. Effects of catalyst distribution, particle geometry, and process conditions on the behavior of a water gas shift reactor under moderate pressures: a modeling study. Reac Kinet Mech Cat 136, 1859–1890 (2023). https://doi.org/10.1007/s11144-023-02431-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02431-x