Abstract
Background
Arylpropionic acid derivatives (APs) are the main triggers of nonsteroidal anti-inflammatory drug (NSAID) hypersensitivity. Data on clinical patterns and risk factors for AP hypersensitivity in children are quite limited.
Aim
To assess the clinical characteristics and potential risk factors for proven AP hypersensitivity in children.
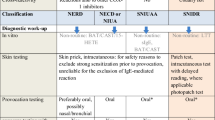
Method
Patients with a history of AP hypersensitivity were retrospectively assessed using a standardized diagnostic algorithm. Children with confirmed hypersensitivity were defined as selective responders or cross-intolerants based on the result of drug provocation tests and further categorized according to the EAACI/ENDA classification. A multivariable logistic regression analysis was performed to analyze the potential risk factors for proven AP hypersensitivity.
Results
A total of 166 patients (51.2% male, median age of six years) with a history of AP hypersensitivity were included. Ibuprofen (89.2%) was the most frequently reported AP in the patients’ histories. The reported hypersensitivity of 40 (22.4%) patients was confirmed by diagnostic testing: eight (13.6%) patients with a history of reaction only to APs and 32 (29.9%) patients with a history of reactions to multiple NSAIDs, including chemically unrelated NSAIDs in addition to APs. Five (12.5%) patients were classified as selective responders and 35 (87.5%) were cross-intolerants. Overall, five (12.5%) of the confirmed cases could not be categorized according to the EAACI/ENDA classification. Older age (aOR: 1.11, 95% CI 1.02–1.21, p = 0.015), chronic urticaria as an underlying disease (aOR: 2.87, 95% CI 1.09–7.54, p = 0.033) and a history of anaphylaxis (aOR: 7.84, 95% CI 1.86–33.04, p = 0.005) were related to confirmed AP hypersensitivity.
Conclusion
Almost a quarter of children and adolescents were confirmed to have AP hypersensitivity. Older age, the presence of chronic urticaria and a history of anaphylaxis were potential risk factors for proven AP hypersensitivity.

Similar content being viewed by others
References
Mori F, Atanaskovic-Markovic M, Blanca-Lopez N, et al. A Multicenter retrospective study on hypersensitivity reactions to nonsteroidal anti-inflammatory drugs (NSAIDs) in children: a report from the European Network on Drug Allergy (ENDA) group. J Allergy Clin Immunol Pract. 2020;8:1022-1031.e1021.
Doña I, Blanca-López N, Cornejo-García JA, et al. Drug hypersensitivity reactions: response patterns, drug involved and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22(5):363–71.
Kowalski ML, Asero R, Bavbek S, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68:1219–32.
Cousin M, Chiriac A, Molinari N, et al. Phenotypical characterization of children with hypersensitivity reactions to NSAIDs. Pediatr Allergy Immunol. 2016;27:743–8.
Cavkaytar O, Arik Yilmaz E, Karaatmaca B, et al. Different phenotypes of non-steroidal anti-inflammatory drug hypersensitivity during childhood. Int Arch Allergy Immunol. 2015;167:211–21.
Guvenir H, Dibek Misirlioglu E, Vezir E, et al. Nonsteroidal anti-inflammatory drug hypersensitivity among children. Allergy Asthma Proc. 2015;36:386–93.
Arikoglu T, Aslan G, Yildirim DD, et al. Discrepancies in the diagnosis and classification of nonsteroidal anti-inflammatory drug hypersensitivity reactions in children. Allergol Int. 2017;66:418–24.
Zambonino MA, Torres MJ, Muñoz C, et al. Drug provocation tests in the diagnosis of hypersensitivity reactions to non-steroidal anti-inflammatory drugs in children. Pediatr Allergy Immunol. 2013;24:151–9.
Jares EJ, Villa RC, Sánchez-Borges M, et al. Drug-Induced anaphylaxis, elicitors, risk factors and management in Latin America. J Allergy Clin Immunol Pract. 2020;8(4):1403-1405.e1.
Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180: 114147.
Cavkaytar O, Arga M. NSAID hypersensitivity in the pediatric population: classification and diagnostic strategies. J Asthma Allergy. 2022;15:1383–99.
Kidon M, Blanca-Lopez N, Gomes E, et al. EAACI/ENDA position paper: diagnosis and management of hypersensitivity reactions to non-steroidal anti-inflammatory drugs (NSAIDs) in children and adolescents. Pediatr Allergy Immunol. 2018;29:469–80.
Blanca-Lopez N, Soriano V, Garcia-Martin E, et al. NSAID-induced reactions: classification, prevalence, impact, and management strategies. J Asthma Allergy. 2019;12:217–33.
Walters KM, Woessner KM. An overview of nonsteroidal antiinflammatory drug reactions. Immunol Allergy Clin North Am. 2016;36:625–41.
Lee Y, Shin YS, Park HS. New phenotypes in hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Curr Opin Allergy Clin Immunol. 2019;19:302–7.
Blanca-Lopez N, Somoza-Alvarez ML, Bellon T, et al. NSAIDs hypersensitivity: questions not resolved. Curr Opin Allergy Clin Immunol. 2018;18:291–301.
Demoly P, Kropf R, Bircher A, et al. Drug hypersensitivity: questionnaire. EAACI interest group on drug hypersensitivity. Allergy. 1999;54:999–1003.
Brockow K, Romano A, Blanca M, et al. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51.
Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs—an ENDA/EAACI drug allergy interest group position paper. Allergy. 2013;68:702–12.
Yilmaz O, Ertoy Karagol IH, Bakirtas A, et al. Challenge-proven nonsteroidal anti-inflammatory drug hypersensitivity in children. Allergy. 2013;68:1555–61.
Ertoy Karagol HI, Yilmaz O, Topal E, et al. Nonsteroidal anti-inflammatory drugs-exacerbated respiratory disease in adolescents. Int Forum Allergy Rhinol. 2015;5:392–8.
Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62:1111–8.
Aberer W, Bircher A, Romano A, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–63.
Bousquet PJ, Gaeta F, Bousquet-Rouanet L, et al. Provocation tests in diagnosing drug hypersensitivity. Curr Pharm Des. 2008;14:2792–802.
Corzo JL, Zambonino MA, Muñoz C, et al. Tolerance to COX-2 inhibitors in children with hypersensitivity to nonsteroidal anti-inflammatory drugs. Br J Dermatol. 2014;170:725–9.
Kidon MI, Liew WK, Chiang WC, et al. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007;144:51–6.
Alves C, Romeira AM, Abreu C, et al. Non-steroidal anti-inflammatory drug hypersensitivity in children. Allergol Immunopathol (Madr). 2017;45:40–7.
Eser Simsek I, Cogurlu MT, Aydogan M. Two approaches for diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity in children. Ann Allergy Asthma Immunol. 2019;123:389–93.
Topal E, Celiksoy MH, Catal F, et al. The value of the clinical history for the diagnosis of immediate nonsteroidal anti-inflammatory drug hypersensitivity and safe alternative drugs in children. Allergy Asthma Proc. 2016;37:57–63.
Minaldi E, Cahill K. Recent updates in understanding NSAID hypersensitivity. Curr Allergy Asthma Rep. 2023;23:181–8.
Doña I, Pérez-Sánchez N, Eguiluz-Gracia I, et al. Progress in understanding hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Allergy. 2020;75:561–75.
Gaffar J, Gabrielli S, Lavine E, et al. Diagnosis of Ibuprofen allergy through oral challenge. Clin Exp Allergy. 2020;50:636–9.
Salas-Casinello M, Sáenz-de Santa María R, López-Sánchez JD, et al. Different patterns of response in hypersensitivity reactions to arylpropionic acid derivatives. J Allergy Clin Immunol Pract. 2023;11(12):3715–23.
Blanca-López N, Pérez-Alzate D, Andreu I, et al. Immediate hypersensitivity reactions to ibuprofen and other arylpropionic acid derivatives. Allergy. 2016;71(7):1048–56.
Blanca-López N, Haroun-Diaz E, Ruano FJ, et al. Acetyl salicylic acid challenge in children with hypersensitivity reactions to nonsteroidal anti-inflammatory drugs differentiates between cross-intolerant and selective responders. J Allergy Clin Immunol Pract. 2018;6(4):1226–35.
Pérez-Sánchez N, Doña I, Bogas G, et al. Evaluation of subjects experiencing allergic reactions to non-steroidal anti-inflammatory drugs: clinical characteristics and drugs involved. Front Pharmacol. 2020;11:503.
Arikoglu T, Tokmeci N, Demirhan A, et al. Evaluation of different protocols for classification of pediatric hypersensitivity reactions to nonsteroidal anti-inflammatory drugs: children with underlying allergic disease should be a separate subgroup. Allergy Asthma Proc. 2024;45(1):14–23.
Hur GY, Park HS. Clinical characteristics of NSAID-induced blended reaction. Allergy Asthma Immunol Res. 2021;13:171–2.
Yilmaz Topal O, Kulhas Celik I, Turgay Yagmur I, et al. Results of NSAID provocation tests and difficulties in the classification of children with nonsteroidal anti-inflammatory drug hypersensitivity. Ann Allergy Asthma Immunol. 2020;125(2):202–7.
Doña I, Barrionuevo E, Salas M, et al. NSAIDs-hypersensitivity often induces a blended reaction pattern involving multiple organs. Sci Rep. 2018;8:16710.
Cavkaytar O, du Toit G, Caimmi D. Characteristics of NSAID-induced hypersensitivity reactions in childhood. Pediatr Allergy Immunol. 2019;30:25–35.
Sánchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for non-steroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000;84:101–6.
Sánchez-Borges M, Capriles-Behrens E, Caballero-Fonseca F. Hypersensitivity to non-steroidal anti-inflammatory drugs in childhood. Pediatr Allergy Immunol. 2004;15(4):376–80.
Hassani A, Ponvert C, Karila C, et al. Hypersensitivity to cyclooxygenase inhibitory drugs in children: a study of 164 cases. Eur J Dermatol. 2008;18(5):561–5.
Podlecka D, Socha-Banasiak A, Jerzynska J, et al. Practical approach to hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Pharmaceuticals (Basel). 2023;16(9):1237.
Imbalzano E, Casciaro M, Quartuccio S, et al. Association between urticaria and virus infections: a systematic review. Allergy Asthma Proc. 2016;37(1):18–22.
Kidon MI, Kang LW, Chin CW, et al. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005;116:e675-680.
Cavkaytar O, Arik Yilmaz E, Buyuktiryaki B, et al. Challenge-proven aspirin hypersensitivity in children with chronic spontaneous urticaria. Allergy. 2015;70(2):153–60.
Pascal M, Muñoz-Cano R, Milà J, et al. Nonsteroidal anti-inflammatory drugs enhance IgE-mediated activation of human basophils in patients with food anaphylaxis dependent on and independent of nonsteroidal anti-inflammatory drugs. Clin Exp Allergy. 2016;46:1111–9.
Matsuo H, Yokooji T, Morita H, et al. Aspirin augments IgE-mediated histamine release from human peripheral basophils via Syk kinase activation. Allergol Int. 2013;62:503–11.
Suzuki Y, Ra C. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: aspirin modulation of IgE-dependent mast cell activation: role of aspirin-induced exacerbation of immediate allergy. J Pharmacol Sci. 2009;110:237–44.
Ensina LF, de Lacerda AE, de Andrade DM, et al. Drug-induced anaphylaxis in children: nonsteroidal anti-inflammatory drugs and drug provocation test. J Allergy Clin Immunol Pract. 2014;2:825.
Ganapathy S, Lwin Z, Ting DH, et al. Anaphylaxis in children: experience of 485 episodes in 1,272,482 patient attendances at a tertiary paediatric emergency department from 2007 to 2014. Ann Acad Med Singap. 2016;45:542–8.
Blanca-López N, Cornejo-García JA, Plaza-Serón MC, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs in children and adolescents: cross-intolerance reactions. J Investig Allergol Clin Immunol. 2015;25:259–69.
Kowalski ML, Makowska JS. Seven steps to the diagnosis of NSAIDs hypersensitivity: how to apply a new classification in real practice? Allergy Asthma Immunol Res. 2015;7(4):312–20.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arikoglu, T., Tokmeci, N., Demirhan, A. et al. Diagnostic evaluation of hypersensitivity reactions to arylpropionic acid derivatives: a descriptive observational study focusing on clinical characteristics and potential risk factors in children. Int J Clin Pharm (2024). https://doi.org/10.1007/s11096-024-01756-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-024-01756-4