Abstract
Background
Several economic studies have assessed the cost-effectiveness of programmed cell death protein-1 (PD-1) inhibitors compared to second-line chemotherapy in treating esophageal squamous cell carcinoma (ESCC). However, there is a lack of economic comparisons among the different PD-1 inhibitors.
Aim
This study aimed to assess the cost-effectiveness of PD-1 inhibitors (nivolumab, pembrolizumab, camrelizumab, and tislelizumab) in second-line treatment for advanced or metastatic ESCC within the Chinese healthcare system.
Method
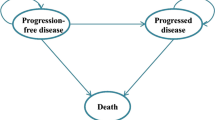
The clinical trials were systematically retrieved from PubMed, Embase, Web of Science, and the Cochrane Library. We established a fractional polynomials model to conduct a network meta-analysis, enabling the calculation of hazard ratios and expected survival rates. Economic outcomes were estimated using a partitioned survival model. The costs and utilities were gathered from published sources. The threshold for willingness-to-pay (WTP) for a quality-adjusted life year (QALY) was set at three times China’s per capita gross domestic product in 2022. Sensitivity analyses (SA) were performed to address uncertainties in the model.
Results
Four phase III randomized controlled trials were included, evaluating the cost-effectiveness of four PD-1 inhibitors, camrelizumab, nivolumab, tislelizumab, and pembrolizumab, compared to chemotherapy for the second-line treatment of advanced or metastatic ESCC. For camrelizumab, nivolumab, tislelizumab, and pembrolizumab, the corresponding incremental cost-effectiveness ratios were $27,375.43/QALY, $205,312.19/QALY, $9,266.73/QALY, and $220,368.10/QALY, respectively. The SA results indicated the robustness of the base analysis findings.
Conclusion
From the Chinese healthcare system, under the WTP of $38,253.48/QALY, tislelizumab is a cost-effective treatment option for the second-line treatment of advanced or metastatic ESCC.



Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
International Agency for Research on Cancer, World Health Organization. https://gco.iarc.fr. Accessed 28 Nov 2023.
Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–73.
Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–67.
Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50(1):12–20.
Shirakawa T, Kato K, Nagashima K, et al. A retrospective study of docetaxel or paclitaxel in patients with advanced or recurrent esophageal squamous cell carcinoma who previously received fluoropyrimidine- and platinum-based chemotherapy. Cancer Chemother Pharmacol. 2014;74(6):1207–15.
Shim HJ, Cho SH, Hwang JE, et al. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. Am J Clin Oncol. 2010;33(6):624–8.
Oh S, Kim E, Lee H. Comparative impact of PD-1 and PD-L1 inhibitors on advanced esophageal or gastric/gastroesophageal junction cancer treatment: a systematic review and meta-analysis. J Clin Med. 2021;10(16):3612.
Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–17.
Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–48.
Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–42.
Shen L, Kato K, Kim SB, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol. 2022;40(26):3065–76.
Chinese Society of Clinical Oncology Guideline Committee. Clinical Practice Guidelines for Esophageal Cancer by the Chinese Society of Clinical Oncology (CSCO). Bei**g: People’s Medical Publishing House. 2022;68-91. ISBN: 9787117329637
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011;11:61.
Petersohn S, McGregor B, Klijn SL, et al. Challenges in conducting fractional polynomial and standard parametric network meta-analyses of immune checkpoint inhibitors for first-line advanced renal cell carcinoma. J Comp Eff Res. 2023;12(8):e230004.
Wu B, Dong B, Xu Y, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS ONE. 2012;7(3):e32530.
National Bureau of Statistics of the People's Republic of China. https://data.stats.gov.cn/easyquery.htm?cn=A01&zb=A010101&sj. Accessed 25 Nov 2023.
Zhang PF, **e D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. 2020;16(17):1189–98.
Liu G, Hu S, Wu J, et al. China guidelines for pharmacoeconomic evaluations. Bei**g: China Market Press; 2020. p. 27–8.
Okada M, Kato K, Cho BC, et al. Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin Cancer Res. 2022;28(15):3277–86.
Information of Drug Winning Bid. https://db.yaozh.com. Accessed 25 Nov 2023.
Zhang Q, Wu P, He X, et al. Cost-effectiveness analysis of camrelizumab vs. placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol. 2021;11:790373.
Li L, Liu X, Huang J, et al. Cost-effectiveness of camrelizumab versus chemotherapy for the treatment of advanced or metastatic esophageal squamous cell carcinoma. J Gastrointest Oncol. 2022;13(1):40–8.
Gong J, Su D, Shang J, et al. Cost-effectiveness of tislelizumab versus docetaxel for previously treated advanced non-small-cell lung cancer in China. Front Pharmacol. 2022;13:830380.
Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–203.
Zargar M, McFarlane T, Chan KKW, et al. Cost-effectiveness of nivolumab in recurrent metastatic head and neck squamous cell carcinoma. Oncologist. 2018;23(2):225–33.
Liu S, Dou L, Wang K, et al. Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front Oncol. 2022;12:899966.
**e Q, Luo Y, Peng X. Cost-effectiveness analysis of pembrolizumab for patients with advanced esophageal cancer at PD-L1 combined positive score≥10. J Comp Eff Res. 2022;11(15):1095–103.
Lang W, Wei J, Jiang Q, et al. Cost-effectiveness analysis of nivolumab versus placebo for relapsed malignant mesothelioma. Int J Clin Pharm. 2023. https://doi.org/10.1007/s11096-023-01662-1.
Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Mak. 2012;32(5):722–32.
Shi F, He Z, Su H, et al. Economic evaluation of tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Front Pharmacol. 2022;13:961347.
Zhan M, Xu T, Zheng H, et al. Cost-effectiveness analysis of pembrolizumab in patients with advanced esophageal cancer based on the KEYNOTE-181 study. Front Public Health. 2022;10:790225.
Liu S, Dou L, Li S. Cost-effectiveness analysis of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal squamous-cell carcinoma in China. Front Pharmacol. 2023;14:1055727.
Acknowledgements
None.
Funding
This study was not supported by project funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Zhao, L., Shi, F. et al. Cost-effectiveness analysis of PD-1 inhibitors as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China: an economic evaluation based on network meta-analysis. Int J Clin Pharm 46, 675–683 (2024). https://doi.org/10.1007/s11096-023-01696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01696-5