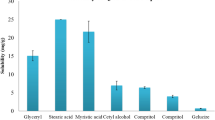
Nanotechnology is emerging as a field in medicine that is expected to elicit significant therapeutic benefits. The main objective of the present study was to formulate and optimize bupropion loaded solid lipid nanoparticles (SLNs) by 23 factorial design so as to improve its bioavailability. The SLNs were prepared by solvent injection method. Eight SLN formulations were obtained by varying the tristearin:soya lecithin (TST:SYL) ratio, amount of drug, and volume of surfactant. Athree-factor, two-level full factorial (23) design was selected to study the interactions of three factors as influencing the drug entrapment efficiency, particle size, and % drug release. The corresponding response surface plots were generated by the Design of Experiment software Version 7.0.0 to visualize simultaneous effects of each variable on the response parameters. The lowest particle size, maximum entrapment efficiency and % drug release from the formulation loaded SLNs at TST:SYL = 1:1 ratio, 75 mg drug content, and 0.1 mL volume of surfactant could be visualized from the response plots and the overall desirability was 0.849. Therefore, SLN2 formulation was considered the best one and the values of independent variables for this formulation were assumed to be optimum for obtaining bupropion loaded SLNs. This lipid exhibited good compatibility with bupropion, without undesired mutual interaction as per differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy data. Relative reduction in diffraction intensities in the x-ray powder diffraction (XRD) studies for the SLNs indicated drug conversion from the crystalline to amorphous form. The in vivo test results showed that the best SLN formulation was significantly different in immobility period, increased percentage of open arm entries and significant increase in number of lines crossed compared to the marketed formulation. Thus, from all the above observations, it was concluded that SLN2 formulation showed improved bioavailability and sustained drug release compared to other formulations, which might be related to increased drug entrapment and surface area and decreased particle size.













Similar content being viewed by others
References
D. Zhang, B. Yuan, M. Qiao, and F. Li, J. Pharm. Biomed. Anal., 33(2), 287–293 (2003).
P. H. Silverstone, R. Williams, L. McMahon, et al., Ann. Gen. Psych., 7(19), 352 – 374 (2008).
R. HMüller, K.Mäder, and S. Gohla, Eur. J. Pharm. Biopharm., 50(1), 161 – 177 (2000).
M. Manchester and P. Singh, Adv. Drug Deliv. Rev., 58(14), 1505 – 1522 (2006).
S. Mukherjee, S. Ray, and R. S. Thakur, Ind. J. Pharm. Sci., 71(4), 349 – 358 (2008).
Houli Li, **aobin Zhao, Yukun Ma, et al., J. Control. Release, 133(3), 238 – 244 (2009).
K. S. Rahul Nair, K. Arun Kumar, T. Vishnu Priya, et al., J. Pharm. Sci. Res., 3(5), 1256 – 1264 (2011).
R. Kumar,M. Yasir, S. A. Saraf, et al., Drug Discov. Today, 5(3), 246–250 (2013).
D. Suvakanta, Murthy P. Narasimha, N. Lilakanta, and Ch. Prasanta, Acta Pol. Pharm., 67(3), 217 – 223 (2003).
P. G. Paterakis, E. S. Korakianiti, P. P. Dallas, and D. M. Rekkas, Int. J. Pharm., 248(1 – 2), 51 – 60 (2002).
R. C. Mashru, V. B. Sutariya, M. G. Sankalia, and P. P. Parikh, Drug. Dev. Ind. Pharm, 31(3), 25–34 (2005).
Bhavin K. Patel, Rajesh H. Parikh, and S. Pooja, J. Drug Deliv., 2013, 1 – 10 (2013).
R. D. Porsolt and A. Bertin, Arch. Int. Pharmacodyn. Ther., 229(2), 327 – 336 (1977).
L. J. Bertoglio, S. R. L. Joca, and F. S. Guimarães, Behav. Brain. Res., 175(1), 183 – 188 (2006).
T. Yamaguchi, A. Tsujimatsu, H. Kumamoto, et al., J. Ethnopharmacol., 143(2), 533 – 539 (2012).
J. W. Moore and H. H. Flanner, Pharm. Tech., 2(5), 64 – 74 (1996).
V. P. Shah, Y. Tsong, and P. Sathe, Pharm. Res, 18(1), 889 – 896 (1998).
K.Westesen, H. Bunjes, and M. H. J. Koch, J. Control. Release, 6(2), 223 – 236 (2005).
G. Abdelbary and R. H. Fahmy, AAPS Pharm. Sci. Tech., 10(1), 211 – 219 (2009).
Z. Rahman, A. S. Zidan, and M. A. Khan, Eur. J. Pharm. Biopharm., 76(1), 127 – 137 (2010).
C. Freitas and R. H. Muller, Eur. J. Pharm. Biopharm, 46(2), 145–151 (1998).
J. Liu, T. Gong, C. Wang, et. al., Int. J. Pharm., 340(1 – 2), 153 – 162 (2007).
K. Manjunath, J. S. Reddy, and V. Venkateswarlu, Meth. Find. Exp. Clin. Pharmacol., 95(3), 127 – 144 (2005).
S. A. Wissing, R. H. Muller, and O. Kayser, Adv Drug Deliv Rev, 56(9),1257–1272 (2004).
Bhavin K. Patel, Rajesh H. Parikh, and S Pooja, J. Drug Deliv., 2013, 1 – 10 (2013).
S. Martins, Eur. J. Pharm. Sci., 45(5), 613 – 623 (2012).
V. Jenning, A. F. Thunemann, and S. H. Gohla, Int. J. Pharm., 199(2), 167 – 177 (2000).
M. Liu, J. Dong, Y. Yang, et al., Eur. Polym. J., 39(7), 375 – 382 (2005).
S. J. Kshirsagar, J. Pharm. Res., 2(9), 1780 – 1785 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swarnalatha, N., Vidyavathi, M. & Rani, J.S. Design, Characterization and Optimization of Solid Lipid Nanoparticles of Bupropion by Using 23 Factorial Design. Pharm Chem J 57, 590–602 (2023). https://doi.org/10.1007/s11094-023-02924-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02924-y