Abstract
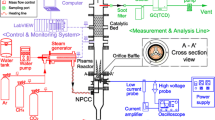
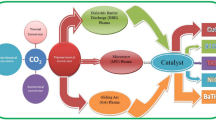
CF4 is commonly used in semiconductor industries, and its removal requires a large amount of energy because it is a highly stable perfluorinated compound. In this study, a rotating arc-catalytic reactor that uses thermal plasma as the heat source for catalysts is introduced as an efficient method for CF4 removal. An AC rotating arc plasma was used with the catalyst for CF4 removal, and the plasma and catalyst were configured serially in this reactor. Destruction and removal efficiency (DRE) of CF4 and the energy efficiency of the removal process were experimentally investigated in the input power range of 2–3 kW and a steam-to-CF4 molar ratio of up to 20. The obtained results suggest that the DRE of CF4 was up to 98% and the energy efficiency was as high as 36.1 g/kWh, which is approximately 3.5 times higher than that obtained using a rotating arc reactor alone. In addition, the thermal efficiency was estimated to be approximately 67%, based on the temperatures of the inlet and outlet gases of the rotating arc-catalytic reactor. Thus, further improvement in the performance can be expected through subsequent thermal management and system optimization. These results confirm that using rotating arc-catalytic reactors is an energy-efficient strategy for application as scrubber systems to remove CF4.













Similar content being viewed by others
References
Mogab CJ, Adams AC, Flamm DL (1978) Plasma etching of Si and SiO2–The effect of oxygen additions to CF4 plasmas. J Appl Phys 49:3796–3803. https://doi.org/10.1063/1.325382
Jeon MH, Ahn C, Kim HU, Kim KN, Lin TZ, Qin H, Kim Y, Lee S, Kim T, Yeom GY (2015) Controlled MoS2 layer etching using CF4 plasma. Nanotechnology 26:355706. https://doi.org/10.1088/0957-4484/26/35/355706
Li P, Zhi D, Zhang X, Zhu H, Li Z, Peng Y, He Y, Luo L, Rong X, Zhou Y (2019) Research progress on the removal of hazardous perfluorochemicals: a review. J Environ Manage 250:109488. https://doi.org/10.1016/j.jenvman.2019.109488
Manash M, Kendrick B (2002) The need for PFC abatement in semiconductor manufacturing inquiry the University of Arkansas undergraduate research. Journal 3:17
Ou Yang CF, Kam SH, Liu CH, Tzou J, Wang JL (2009) Assessment of removal efficiency of perfluorocompounds (PFCs) in a semiconductor fabrication plant by gas chromatography. Chemosphere 76:1273–1277. https://doi.org/10.1016/j.chemosphere.2009.06.039
EPA US (2016) Inventory of U.S. Greenhouse gas emissions and sinks: 1990–2014, https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks
Jia L, & Ma S (2005) The experimental study on high temperature air combustion and CF4 decomposition. In: Heat transfer summer conference, p 705-708
Qin L, Han J, Wang G, Kim HJ, Kawaguchi I (2010) Highly efficient decomposition of CF4 gases by combustion. In: Conference on environmental pollution and public health, p 126–130
Takita Y, Morita C, Ninomiya M, Wakamatsu H, Nishiguchi H, Ishihara T (1999) Catalytic decomposition of CF4 over AIPO4-based catalysts. Chem Lett 28:417–418. https://doi.org/10.1246/cl.1999.417
Jeon JY, Xu XF, Choi MH, Kim HY, Park YK (2003) Hydrolytic decomposition of PFCs over AIPO4-Al2O3 catalyst. Chem Commun (Camb) 11:1244–1245. https://doi.org/10.1039/b302064a
El-Bahy ZM, Ohnishi R, Ichikawa M (2003) Hydrolysis of CF4 over alumina-based binary metal oxide catalysts. Appl Cat B 40:81–91. https://doi.org/10.1016/S0926-3373(02)00143-1
Xu X-F, Jeon JY, Choi MH, Kim HY, Choi WC, Park Y-K (2007) The modification and stability of ℽ–Al2O3 based catalysts for hydrolytic decomposition of CF4. J Mol Cat A Chem 266:131–138. https://doi.org/10.1016/j.molcata.2006.10.026
Song J-J, Chung S-H, Kim M-S, Seo M-G, Lee Y-H, Lee K-Y, Kim J-S (2013) The catalytic decomposition of CF4 over Ce/Al2O3 modified by a cerium sulfate precursor. J Mol Cat A Chem 370:50–55. https://doi.org/10.1016/j.molcata.2012.12.011
Anus A, Sheraz M, Jeong S, Kim E, Kim S (2021) Catalytic thermal decomposition of tetrafluoromethane (CF4): a review. J Anal Appl Pyrol 156:105126
Sun J-W, Park DW (2003) CF4 Decomposition by thermal plasma processing. Korean J Chem Eng 20:476–481. https://doi.org/10.1007/BF02705551
Kim D, Park DW (2008) Decomposition of PFCs by steam plasma at atmospheric pressure. Surf Coatings Technol 202:5280–5283. https://doi.org/10.1016/j.surfcoat.2008.06.023
Park H-W, Choi S, Park D-H (2013) Effect of reaction gases on PFCs treatment using arc plasma process. Clean Technol 19:113–120. https://doi.org/10.7464/ksct.2013.19.2.113
Ming Lee HM, Chen SH (2017) Thermal abatement of perfluorocompounds with plasma torches. Energy Procedia 142:3637–3643. https://doi.org/10.1016/j.egypro.2017.12.256
Chen S-H, Živný O, Mašláni A, Chau S-W (2019) Abatement of fluorinated compounds in thermal plasma flow. J Fluor Chem 217:41–49. https://doi.org/10.1016/j.jfluchem.2018.10.004
Park H-W, Cha WB, Uhm S (2018) Highly efficient thermal plasma scrubber technology for the treatment of Perfluorocompounds (PFCs). Appl Chem Eng 29:10–17
Moon G-H, Kim J-Y (2019) Study on treatment characteristics of perfluorinated compounds using a high temperature plasma. Appl Chem Eng 30:108–113
Kim K-T, Song Y-H, Cha MS, Lee DH, Kim D-H (2008) Characteristics of rotating arc for CF4 removal. Int J Environ Waste Manag 2:412–422
Kim Y, Kim K-T, Cha MS, Song Y-H, Kim SJ (2005) CF 4 Decompositions using streamer-and glow-mode in dielectric barrier discharges. IEEE Trans Plasma Sci 33:1041–1046
Yu SJ, Chang MB (2001) Oxidative conversion of PFC via plasma processing with dielectric barrier discharges. Plasma Chem Plasma Process 21:311–327. https://doi.org/10.1023/A:1011066208188
Na BK, Choi J-W, Lee H, Song HK (2002) Decomposition of tetrafluorocarbon in dielectric barrier discharge reactor. Korean J Chem Eng 19:917–920. https://doi.org/10.1007/BF02707211
Chang MB, Lee HM (2004) Abatement of perfluorocarbons with combined plasma catalysis in atmospheric-pressure environment. Cat Today 89:109–115. https://doi.org/10.1016/j.cattod.2003.11.016
Pan KL, Chen YS, Chang MB (2019) Effective removal of CF4 by combining nonthermal plasma with ℽ–Al2O3. Plasma Chem Plasma Process 39:877–896. https://doi.org/10.1007/s11090-019-09990-9
Cui Z, Zhang X, Yuan T, **ngya P, Luo Y, Tang J (2020) Plasma-assisted abatement of SF6 in a dielectric barrier discharge reactor: investigation of the effect of packing materials. J Phys D: Appl Phys 53:025205. https://doi.org/10.1088/1361-6463/ab4ba7
Lim MS, Kim SC, Chun YN (2011) Decomposition of PFC gas using a water jet plasma. J Mech Sci Technol 25:1845–1851. https://doi.org/10.1007/s12206-011-0422-z
Lim MS, Chun YN (2017) Decomposition characteristics of tetrafluoromethane using a waterjet plasma scrubber. J Clim Change Res 8(1):63. https://doi.org/10.15531/ksccr.2017.8.1.63
Muthuraman G, Ramu AG, Cho YH, McAdam EJ, Moon IS (2018) Sustainable degradation of carbon tetrafluoride to non-corrosive useful products by incorporating reduced electron mediator within electro-scrubbing. J Ind Eng Chem 63:275–280. https://doi.org/10.1016/j.jiec.2018.02.025
Tsai CH, Kuo ZZ (2009) Effects of additives on the selectivity of byproducts and dry removal of fluorine for abating tetrafluoromethane in a discharge reactor. J Hazard Mater 161:1478–1483. https://doi.org/10.1016/j.jhazmat.2008.04.118
Lee B, Kim S, Song J, Ryi S-K, Lim H (2018) Conceptual design of a new SF6 abatement technology using a multi-bed series reactor for the production of valuable chemicals free of toxic wastes. Energy Sci Eng 6:73–82. https://doi.org/10.1002/ese3.183
Chung JK, Lee KY, Lee SG, Lee EM, Mo SH, Lee DK, Kim SG (2017) The development of scrubber for F-gas reduction from electronic industry using pressure swing adsorption method and porous media combustion method. Clean Technol 23:181–187
S Gordon and BJ McBride (1994) Computer program for calculation of complex chemical equilibrium compositions and applications. NASA Ref Publ 1311
Fridman A (2008) Coal conversion in non-thermal plasma. In: Fridman A (ed) Plasma chemistry. Cambridge University Press, New York
Gutsol A, Rabinovich A, Fridman A (2011) Combustion-assisted plasma in fuel conversion. J Phys D Appl Phys 44:274001. https://doi.org/10.1088/0022-3727/44/27/274001
Jo S, Lee DH, Song Y-H (2013) Effect of gas temperature on partial oxidation of methane in plasma reforming. Int J Hydr Energy 38:13643–13648. https://doi.org/10.1016/j.ijhydene.2013.08.049
Puresphere technical data sheet, PFCs decomposition catalyst of purelyst PFC-101, http://www.puresphere.co.kr/e_sub0201/product/article_view/id/52
Lee DH, Kim K-T, Cha MS, Song Y-H (2007) Optimization scheme of a rotating gliding arc reactor for partial oxidation of methane. Proc Combust Inst 31:3343–3351
Narengerile SH, Saito H, Watanabe T (2010) Decomposition mechanism of fluorinated compounds in water plasmas generated under atmospheric pressure. Plasma Chem Plasma Process 30:813–829. https://doi.org/10.1007/s11090-010-9259-y
Acknowledgements
Dr. Sungkwon Jo and Mr. Donghyun Cho contributed equally to this work. The authors acknowledge the financial support received from the Ministry of Science and ICT of the Republic of Korea (Grant Number NK236F).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, S., Cho, D., Lee, D.H. et al. Investigation of Rotating Arc-Catalytic Reactor for CF4 Removal with High Energy Efficiency. Plasma Chem Plasma Process 42, 1311–1327 (2022). https://doi.org/10.1007/s11090-022-10274-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10274-y