Abstract
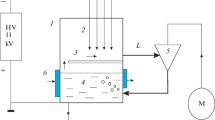
Electron beam (EB) technology has an advantage for treating dilute environmental pollutants in gases due to high-density population of active species such as radicals and atoms. In general, OH radicals play an important role of initiating the decomposition and removal of such pollutants. It is quite important to understand the behavior of OH radical production for the development of efficient decomposition/removal processes and the comparison with other purification methods. The number of OH radicals produced in humid N2 at doses of 2.0–10.0 kGy with dose rates of 0.17–2.55 kGy/s under 1-MeV EB irradiation was indirectly determined using an index of oxidation of CO to CO2, which has been used in atmospheric chemistry. An experiment under conditions where all OH radicals produced react with CO demonstrated that the concentration of CO2 increased linearly with doses of 0–10 kGy, and the G(OH) was estimated as 4.90.





Similar content being viewed by others
References
Chmielewski AG, Tyminski B, Dobrowolski A, Iller E, Zimek Z, Licki J (2000) Radiat Phys Chem 57:527–530
Doi Y, Nakanishi I, Konno Y (2000) Radiat Phys Chem 57:495–499
Hakoda T, Hashimoto S, Fujiyama Y, Mizuno A (2000) J Phys Chem A 104:59–66
Sun Y, Hakoda T, Chmielewski GA, Hashimoto S, Zimek Z, Bulka S, Ostapczuk A, Nichipor H (2001) Radiat Phys Chem 62:353–360
Hakoda T, Hashimoto S, Kojima T (2002) Bull Chem Soc Jpn 75:2177–2183
Hirota K, Woletz K, Paur HR, Mätzing H (1995) Radiat Phys Chem 46:1093–1097
Hirota K, Hakoda T, Arai H, Hashimoto S (2000) Radiat Phys Chem 57:63–73
Hirota K, Sakai H, Washio M, Kojima T (2004) Ind Eng Chem Res 43:1185–1191
Hirota K, Hakoda T, Taguchi M, Takigami M, Kim H, Kojima T (2003) Environ Sci Technol 37:3164–3170
Willis C, Boyd AW (1976) Int J Radiat Phys Chem 8:71–111
Mätzing H (1991) Adv Chem Phys 80:315–402
Sander SP, Finlayson-Pitts BJ, Friedl RR, Golden DM, Huie RE, Keller-Rudek H, Kolb CE, Kurylo MJ, Molina MJ, Moortgat GK, Orkin VL, Ravishankara AR, Wine PH (2006) Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies Evaluation Number 15
Campbell MJ, Sheppard JC, Au BF (1979) Geophys Res Lett 6:175–178
Campbell MJ, Farmer JC, Fitzner CA, Henry MN, Sheppard JC, Hardy RJ, Hopper JF, Muralidhar V (1986) J Atmos Chem 4:413–427
Forster R, Frost M, Fulle D, Hamann HF, Hippler H, Schlepegrell A, Troe J (1995) J Chem Phys 103:2949–2958
Sugawara K, Ishikawa Y, Sato S (1980) Bull Chem Soc Jpn 53:1344–1351
Bohn B, Zetzsch C (1998) J Chem Soc Faraday Trans 94:1203–1210
Stedman DH, Niki H (1973) Environ Lett 4:303–310
Su Z-Z, Ito K, Takashima K, Katsura S, Onda K, Mizuno A (2002) J Phys D: Appl Phys 35:3192–3198
Prasad SS, Huntress WT (1980) Astrophys J Suppl Ser 43:1–35
Rowe BR, Vallee F, Queffelec JL, Gomet JC, Morlais M (1988) J Chem Phys 88:845–850
Jones FT, Sworski TJ (1966) J Phys Chem 70:1546–1552
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J (2004) Atmos Chem Phys 4:1461–1738
Howard MJ, Smith IWM (1980) Chem Phys Lett 69:40–44
Barnett AJ, Marston G, Wayne RP (1987) J Chem Soc Faraday Trans 2 83:1453–1463
Acknowledgments
The authors thank Dr. Hyun-Ha Kim, National Institute of Advanced Industrial Science and Technology, for useful comments on the determination of OH radical number.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hakoda, T., Shimada, A., Matsumoto, K. et al. Determination of the Number of OH Radicals in EB-Irradiated Humid Gases Using Oxidation of CO. Plasma Chem Plasma Process 29, 69–78 (2009). https://doi.org/10.1007/s11090-008-9162-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-008-9162-y