Abstract
High temperature corrosion and slag deposition significantly reduce the thermal efficiency and lifespan of biomass-fired boilers. Surface modification with protective coatings can enhance boiler performance and prevent commercial losses due to maintenance and damage. This review focuses on the development of corrosion-resistant coatings (CRCs) and anti-slagging coatings (ASCs) over the past decade. CRCs are explored through thermal spray processes that include arc spray, atmospheric plasma spray (APS), high-velocity oxygen fuel (HVOF), detonation gun (D-gun™), and cold spray. Studies on alloys, ceramics, and ceramic–metal composites are summarised, highlighting the high temperature corrosion prevention mechanisms and discussing new coating materials. ASCs are reviewed in the context of advancements via thermal spray and slurry spray methods. The mechanisms for slag reduction, testing methods to evaluate ASC effectiveness, and the necessary architecture for preventing slag deposition are examined. A lab-based rig simulating fly ash deposition onto water-cooled coating coupons for anti-slagging investigations is also presented. Further research is needed to develop and evaluate materials for ASCs effectively.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Slag deposition and high temperature corrosion on heat exchanger surfaces are major drawbacks of industrial boilers that fire solid fuels such as biomass and/or coals. Slag deposits contain corrosive alkali salts, released from solid fuel combustion, which cause high temperature corrosion to the metal surface [1]. Fly ash that has high amounts of chlorine (Cl) and sulphur (S) increases the risk of corrosion failure on the boiler components [2]. A combination of alkali salts in deposits results in low-melting point eutectics that severely corrode boiler steels at temperatures lower than the melting point of each individual salt [3, 4].
Figure 1a shows a schematic diagram of crucial boiler components that are prone to high temperature corrosion and degradation during normal operations. The vulnerable areas include the combustion zone, waterwalls, reheaters, and economiser [5]. Figure 1b illustrates common types of slag deposits found on boiler tubes. These deposits typically form on the leeward side of the boiler tubes [6]. Slag deposits reduce thermal efficiency of boilers because of the thickness increase between the heat source and water inside waterwall or heat exchanger tubes, and energy is also wasted to heat up the slag instead of water behind the heat exchanger surface. The slag deposition and high temperature corrosion decrease the performance and shorten the lifespan of boilers.
The boiler performance impacts financial outcomes of industries. For example, Sugarcane Research Australia estimates that it costs approximately five million dollars yearly for the Australian sugarcane mill industry to repair and cover the cost of downtime of inefficient boiler operations [4]. These challenges can be addressed by understanding the slag formation mechanisms in boilers as well as the corrosion attack on boiler steels by the slag deposits. Appropriate technical and engineering countermeasures may then be applied accordingly to minimise both issues.
Slag Deposition Mechanisms in Boilers
Slag is an unwanted material from combustion process. Slag adheres firmly on heat exchanger surface of boilers, which affects performance and thermal efficiency of the boilers [6]. Niu et al. [1] summarises slag formation mechanisms in biomass-fired boilers that are shown in Fig. 2. Firstly, biomass fuels are combusted. During the combustion of biomass, three major products are generated: (a) alkali salt aerosols, (b) biomass ash, and (c) gaseous products. Next, depending on the starting materials and reactions among them, the products formed from biomass combustion include alkali-induced slagging, silicate melt-induced slagging, and other by-products such as agglomerated ash and fly ash.
Formation mechanisms of alkali-induced slag, silicate melt-induced slag, agglomerated ash, and fly ash in biomass-fired boilers, adapted and redrawn from [1]
Alkali-induced slagging: The alkali salt aerosols condense onto the heating surface of boilers to form a “sticky initial layer”. The condensing mechanisms of the alkali salts have been studied by researchers [7,8,9]. In the meantime, alkali salt aerosols can also condense on ash particles, forming a sticky initial layer on the ash surface. The first sticky layer on the boiler tube surface captures more coarse ash particles until the adhesion force is inadequate to capture more coarse ash. Then, fine ash particles rich in sticky alkali salts adhere to the previously formed coarse ash layer. This forms a layer of fine ash particles until the adhesion force is enough to recapture coarse ash particles. Formation of these coarse and fine ash layers occurs repeatedly, resulting in alternating layers of biomass ash [10]. This process forms alkali-induced slags on the heat exchanger surface in boilers.
Agglomerated ash: This issue is closely related to alkali-induced slagging. Alkali salts chemically react with silicon dioxide of ash particles to form molten or partially molten eutectic silicate materials. These silicates result in the adhesion and agglomeration of ash particles. The agglomerated ash is generally an operational issue for fluidised bed boilers because heavy agglomerated particles interrupt the fluidisation of the biomass fuels. Thus, it may not directly or significantly contribute to the formation of slag on heat exchanger surfaces of boilers.
Silicate melt-induced slagging: This type of slag is also known as ash fusion. It is formed when the operating temperature of boilers exceeds the melting point of biomass ash. Alkali salts can react with the alumina and silica of ash and form molten aluminosilicate materials. Depending on the compositions of biomass fly ashes, their melting point could range from 1100 to 1300 °C [11]. Molten ash deforms and adheres on impacting the surface of boiler heat exchangers. Thus, if the operating temperature of boilers facilitates ash fusion, the heat alone is enough to melt ash particles, leading to melt-induced slagging.
Fly ash formation: Biomass ash that has not been transformed into alkali-induced slag, agglomerated ash, or silicate melt-induced slag is carried away by flue gas during combustion in the form of fly ash.
High Temperature Corrosion by Slag Deposits on Boiler Steels
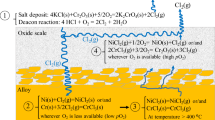
High temperature corrosion on boiler components takes place underneath the slag deposit [1]. The amount of corrosive content in slag deposits or fly ash depends on the fuels combusted in the boilers. Fly ash that exhibits a high percentage of chlorine and sulphur leads to high risk of corrosion failure to boiler components [2]. Therefore, only corrosion mechanisms by both alkali chlorides and alkali sulphates are explained in Fig. 3.
A schematic of high temperature corrosion mechanisms on boiler tubes by ash deposits containing chlorine and sulphur representing: a an early stage, b corrosion on oxidation scale by the corrosive deposit and crack formation, c active oxidation and crack propagation, and d formation of iron chloride and iron sulphide underneath oxidation and corrosion scales, adapted and redrawn from [12]
Alkali chlorides: In biomass-fired boilers, alkali chlorides are usually found at the interface of boiler steel and slag deposits [10, 13]. The alkali chlorides initiate chlorine-induced corrosion on boiler steels under thermodynamically favourable conditions, followed by active oxidation by chlorine. An active oxidation mechanism of high temperature corrosion caused by slag deposits that contain chlorine species is explained by Grabke and Krumm [12, 14]. The corrosion mechanism is summarised in Fig. 3. Firstly, in Fig. 3a, condensation of alkali salts takes place on the oxidation scale of steel. The corrosion is minimal at this stage as the partial pressure of oxygen is high, which slows down the formation of magnetite (Fe3O4) and hematite (Fe2O3). Then, in Fig. 3b, reactions between the deposited salts and oxidation scale begins; i.e., chlorine attacks iron, which is present in the oxidation scale, and forms volatile metal chloride (FeOCl). In addition, Fe3O4 of the oxidation scale could locally transform into Fe2O3, which leads to crack formation. Next, in Fig. 3c, the cracks in the oxidation scale allow diffusion of corrosive chlorine gas (Cl2) from the surrounding environment to reach the metal surface underneath the oxidation scale. Chlorine gas could, again, react with metal oxide to form FeOCl when the partial pressure of oxygen (O2) is low. Active oxidation by chlorine takes place at this stage. Reaction of Cl2 with the base metal forms an easily vaporised iron chloride (FeCl2). This iron chloride is further oxidised to form Fe2O3 or Fe3O4 on the top surface of the oxidation scale and releases Cl2 in the process. The released Cl2 re-enters the loop of reactions and acts as a catalyst to produce more corrosion products. This type of corrosion is typified by potassium chloride (KCl) deposits, whereby KCl causes severe damage to stainless steel that forms protective chromium oxide (Cr2O3) scales [3, 15, 16]. A similar phenomenon was reported by Kung et al. [17] exploring high temperature corrosion mechanisms for iron and nickel-based alloys in sulphur and chlorine-containing environments for coal-fired boilers. The authors explained that the “active sulphide-to-oxide corrosion mechanism” accounts for the rapid corrosion of low-alloy ferritic steels and lower-grade stainless steels, attributed to the formation of iron chloride (FeCl2) vapour and the resulting cyclic reactions.
Alkali sulphates: Despite the presence of corrosive sulphur dioxide (SO2), it was reported that SO2 was unable to diffuse through the oxidation scale to react with the boiler steel surface underneath unless there are cracks in the metal oxide scale [14]. The crack formation may be introduced by corrosion mechanisms as described in Fig. 3b–c. Then, it becomes possible for SO2 to reach the bare metal surface through cracks and contribute to further corrosion. When the partial pressure of O2 is low, the previously formed FeCl2 could react with SO2 and form iron sulphide (FeS) as shown in Fig. 3d. Although sulphate does not significantly contribute to corrosion damage, the combination of alkali sulphate and chloride salts could form eutectic mixtures that may severely corrode boiler steels. For example, potassium sulphate (K2SO4) and sodium sulphate (Na2SO4) have been reported to cause insignificant damage after high temperature corrosion exposure if the protective oxidation scale of boiler steel remains intact and protective [18, 19]. However, alkali sulphates and chlorides could form low-melting point mixtures and then cause severe corrosion. A mixture of K2SO4 and KCl has a melting point of 690 °C [20], while each individual alkali salt melting point is 1069 °C and 774 °C, respectively. This mixture could cause more severe corrosion damage compared to the individual alkali salts [21, 22].
Current Methods to Minimise Slag Deposition and Corrosion in Boilers
The slag deposition and high temperature corrosion issues are closely related and, occasionally, one technical countermeasure could solve both challenges. Thus, to minimise slag deposition, additives could be applied to solid fuels to alter chemical compositions and/or increase the melting point of the fly ash, which may reduce the slagging tendency. For example, sulphur-based additives [23] and silicate-based additives [24] were combusted with solid fuels in boilers to alter chemical compositions of slags and consequently increased their melting points via sulfation and formation of high-melting point alkali silicates, respectively. The additives create higher melting point slags that reduce the tendency of slag deposition [25, 26].
Another common countermeasure is to pre-treat the fuels and remove slag-forming elements from the raw biomass. Fuel pre-treatment is executed by washing raw biomass with water or pickling in acid to eliminate slag-forming elements such as alkali metals and chlorine. However, pre-treated biomass fuel must be dried before the combustion process in boilers, which requires additional processing and energy [27]. These countermeasures require a constant supply of additional additives, fuels, and energy that add extra logistic complexity and boiler operation cost. On the other hand, protective coatings may be regarded as a long-term solution to address both slag deposition and corrosion issues in boilers.
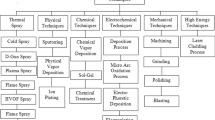
This paper overviews development on corrosion-resistant coatings, followed by anti-slagging coatings for boiler applications in the past decade. Although there are several techniques to produce coatings to address high temperature corrosion in boilers, thermal spray is a widely used process to fabricate protective coatings for boiler components. Therefore, only thermal spray processes that have been used to develop corrosion-resistant coatings are discussed. However, anti-slagging coatings—a much less explored area compared to corrosion-resistant coatings— and their recent development, investigates reduction mechanisms.
The research gaps are related to the processing and material formulation of anti-slagging coatings and appropriate evaluation techniques that explore industrial applications. A thermal spray coating architecture to resist slag deposition without compromising the ability to withstand high temperature corrosion in boilers is proposed. In addition, a rig to mimic slag deposition using a flame spray method to artificially deposit slag on water-cooled coupons for further anti-slagging assessment of the coatings is presented.
Corrosion Resistant and Anti-slagging Coatings for Boilers
Corrosion-resistant coatings have been developed using laser cladding, weld-overlay, diffusion coating methods, slurry spray, and thermal spray processes. These coating fabrication processes are summarised in Table 1, and only non-thermal spray processes are further reviewed in Sect. “Corrosion-Resistant Coatings Prepared by Non-thermal Spray Processes”. Deposition of corrosion-resistant coatings on large boiler parts using conventional routes is challenging. Corrosion-resistant coatings deposited by thermal spray processes, backed up by advancements over the past decade, are explored in Sect. “Corrosion Resistant Coatings Prepared by Thermal Spray Processes”. Anti-slagging coating developments in the past decade and coating performance evaluation are highlighted and discussed from Sect. “Anti-slagging Coatings for Boilers” to Sect. “Methods to Evaluate Slag Resistance of Coatings for Boiler”.
Electron backscatter diffraction inverse pole map**s, grain size distribution, and distribution of boundary misorientation angle of a1–a3 conventional laser cladding, and b1–b3 EHLA 4%wt TiC—96wt% IN625, adapted from [44] with labels added
Weld Overlays
Weld overlays, depending on their functionalities, can be classified into four types: weld cladding, hardfacing, buildup, and buttering. Weld cladding is a process to deposit a thick layer of a corrosion-resistant material on a low alloy substrate. Hardfacing is performed to achieve a wear resistance/abrasion/erosion resistance top layer to a metal surface. Buildup, on the other hand, is used to refurbish worn workpieces to their original shapes. Buttering is a process to facilitate welding of dissimilar metals [29]. Weld overlay coatings are commonly deposited by shielded metal arc welding (SMAW), gas metal arc welding (GMAW), or gas tungsten arc welding (GTAW). There are many welding techniques to produce weld overlay coatings. However, a welding process must be selected on the basis of the filler materials, the coating-substrate compatibility, or the overlay properties for specific applications [45].
Figure 7 illustrates the GTAW process of weld overlay coating production. In this process, an arc is generated between the tungsten inert gas (TIG) tip and the metal substrate to create a weld pool as well as to melt the filler material, which later cools down to form a weld overlay coating. For example, Inconel 625 (58 Ni, 20-23 Cr, 8-10 Mo, 5 Fe) is deposited on boiler steels [46,47,48] where corrosion resistance is conferred by the formation of nickel and chromium oxides [47]. Nevertheless, the dilution of substrate materials can range from 5 to 20% [28]. Dilutions less than 5% may cause issues with bonding of the overlay to the substrate. On the other hand a dilution higher than 15% becomes uneconomical due to the cost increase of the filler material [29]. Welding speed is a key parameter to control the dilution where slow travel speed causes high dilution [29]. In a similar fashion to conventional laser cladding processes, the weld overlay speed can cause challenges in producing coatings for large boiler components.
Schematic diagram of gas tungsten arc welding (GTAW) process [45]
Diffusion Process Coatings
Pack cementation is a conventional method to deposit diffusion coatings. In this process, Fig. 8, workpieces are placed inside a heated retort containing a donor alloy under an inert or hydrogen atmosphere. The donor alloy then releases solute material to form a diffusion coating underneath the original surface of the workpieces [49]. Once the diffusion coating formed on a substrate, it provides a new functionality to the coated material. For instance, aluminium diffusion coatings provide high temperature oxidation resistance due to the formation of aluminium oxide, and diffused silicon coatings enable wear resistance since the formation of silica in an oxidising environment promotes high hardness and corrosion resistance [50].
Schematic diagram of a pack cementation process to deposit diffusion coatings, adapted and redrawn from [49]
Diffusion coatings could also be applied to provide boiler components protection against high temperature corrosion. Diffusion coatings are deposited onto targeted surfaces by either directly heating the coating materials on the substrate in an inert atmosphere or heating the substrate in the environment of vaporised coating materials [51].
Aluminised coatings have reduced the corrosion rate in gaseous environments similar to those experienced under biomass combustions [52]. However, the coating failed to provide corrosion resistance against corrosive KCl in a simulated boiler environment and consequently was not recommended for crucial boiler components [53]. Figure 9 shows damage on an aluminised coating prepared by a diffusion slurry method on P91 boiler steel (ASTM A335 Type 2 equivalent) after high temperature corrosion by KCl deposits. The degradation of the aluminised coating was the result of active oxidation that arose when corrosive chlorine species reacted with aluminium, iron, and chromium of the aluminised specimen, forming corrosion products that migrated to coating surface. Corrosion evidence was indicated by the migration of aluminium and iron towards the coating surface and consequently this led to porosity at the location where these elements were prior to corrosion. Corrosive KCl was also detected in the coating in the coating porous area. Therefore, it was recommended that aluminised coatings should not be used in corrosive boiler environments in the presence of chlorine-containing salts. The application of diffusion coatings for boilers also has technical drawbacks such as thin coating thicknesses, possible inconsistency of coating composition, and the risk of localised corrosion due to coating defects [28].
a Cross-sectioned SEM image of an aluminised P91 boiler steel after high temperature corrosion by KCl deposit at 560 °C for 336 h showing (1) epoxy resin, (2) aluminium oxide, (3) hematite, (4) aluminium oxide, (5) aluminium-iron, and (6) P91 substrate. b X-ray map** of elements in the area in (a) marked with rectangular box, adapted and rescaled from [53]
Slurry Spray Coatings
Slurry spray is a low-cost approach to deposit corrosion-resistant coatings onto surfaces of boiler components. Figure 10 illustrates traditional slurry spray coating deposition. Generally, coating powders are dispersed in liquid binder materials and dispersants to form a slurry, which can be sprayed onto components to form wet coatings. Subsequently, drying and sintering are required to securely bond coatings onto the substrates [54, 55]. Pressure stam** prior to sintering can add the densification and bonding mechanisms. Hexagonal boron nitride (h-BN) is a high temperature material of interest due to its high thermal conductivity, inertness, and lubricant characteristics [56]. A h-BN slurry spray coating has impeded the penetration of corrosive gases and prevented corrosion of the underlying metal [57].
Sequence of conventional air spray coating deposition process. a Slurry preparation, b spraying multi-layers of coating with an air spray gun, c drying the solvent, d stam** with pressure, and e sintering, adapted and redrawn from [58]
However, there are drawbacks for slurry spray coating processes. Firstly, coating materials such as sodium silicate could reabsorb moisture prior to the sintering process [31]. The moisture absorption may lead to wet coating deformation and poor coating integrity prior to sintering. Secondly, the sintering temperature affects the microstructure and performance of slurry spray coatings [62]. Studies on arc sprayed coatings for high temperature corrosion in boilers are summarised in Table 2.
Schematic diagram of a twin wire arc spray (TWAS) process, adapted and redrawn from [63]
Table 2 indicates that the corrosion resistance has exploited nickel–chromium and other chromium-containing alloys as coating materials. The presence of chromium contributes to the formation of a protective Cr2O3 scale against high temperature corrosion from alkali salts. Especially, high chromium content arc sprayed coating such as Ni-50Cr (at%) showed superior protection to corrosion [66]. The high chromium content serves as a source for forming a Cr2O3 layer, which prevents corrosive species from diffusing to and corroding the metal surface beneath the coating. Similarly, protective oxides and spinels from alloying elements provide protection against high temperature corrosion [64, 68, 69].
Additionally, minor elements such as boron (B), titanium (Ti), and molybdenum (Mo), as well as the microstructures of the arc sprayed coating, influence the corrosion resistance. Wang et al. [67] studied the influence of boron in arc sprayed NiCr coatings on corrosion resistance against a molten salt environment. The addition of boron promoted the formation of oxides that reduced the scale thickness. Nevertheless, excessive boron content led to brittle coatings, which increased the risk of crack formation and accelerated corrosion. The optimum amount of 1–2 wt% boron improved the corrosion resistance of coatings. Qin et al. [65] investigated high temperature corrosion on exceptionally dense NiCrTi and NiCrMo coatings deposited by an arc spray method equipped with a modified convergent nozzle. This modified nozzle allowed high velocity sprayed particles up to 152 ± 26 m/s, resulting in anisotropic splats with protective oxide boundaries and a coating porosity below 2%. The addition of trace element titanium promoted the enrichment of chromium along splat boundaries, and molybdenum improved stability of protective Cr2O3 formed on the boundaries. Consequently, the combination of high dense coating microstructures and stabilised protective oxides along the splat boundaries provided an effective corrosion resistance against molten salt environments.
The arc spray process is not limited to electrical conducting wires since ceramic materials have been introduced as a core material to improve the mechanical properties of the coatings. Cheng et al. [70] introduced chromium carbide (Cr3C2) as a core material for 96 wt% FeCrNiAlMnB—12 wt% Cr3C2 feedstock using the high velocity arc spray (HVAS) technique. The coating formed a protective oxide layer to resist high temperature corrosion caused by salt mixture deposits. Apart from corrosion resistance against salt deposits, the coating showed a high hardness of 600–800 HV0.3 due to the formation of hard phases such as Cr7C3. As well, the presence of a hard carbide such as Cr3C2 improved the erosion resistance [71], which would be beneficial in prolonging the lifespan of boilers.
Atmospheric Plasma Spray (APS) Coatings
Atmospheric plasma spray (APS) is a thermal spray process that uses an electric arc and plasma gases to generate a plasma jet capable of melting and propelling powder feedstocks to form a coating on substrates. A schematic diagram describing APS process is presented in Fig. 13. Firstly, an electric arc is generated between the anode and cathode. It is elongated and stabilised within the cascading neutrode. The plasma flame is generated when the primary gas (argon) and secondary gases (nitrogen or hydrogen) are injected and ionised by an electric arc between two electrodes to generate a plasma jet flame [72]. APS could be used to deposit both ceramic and metallic coatings owing to the plasma flame temperature of up to 15,000 °C [61, 62]. A powder feedstock is carried to the plasma flame with a carrier gas, which then gets melted and propelled to a substrate where it impacts, cools rapidly and forms a coating.
APS has been used to deposit ceramic coatings for applications such as yttria-stabilised zirconia (YSZ) to serve as a thermal barrier on gas turbine blades [73]. Hafnium diboride (HfB2) is another example for ultra-high temperature applications, i.e. the surface of supersonic aircrafts [74]. APS coatings can also be applied to the surface of industrial boiler components to provide protection against harsh and corrosive environments at elevated temperature. The materials for APS coatings include alloys and ceramic oxides, see Table 3. The corrosion-resistant APS coatings in boilers employ alloy coatings that form protective oxide scales, whereas ceramic oxide coatings are chemically inert and act as barriers between metal boiler components and the corrosive environment.
Corrosion-Resistant Alloy APS Coatings
As summarised in Table 3, APS has been widely used to develop high temperature corrosion-resistant alloy coatings for applications in boilers. Nickel-based alloys [75,76,77, 83] are common materials that have been deposited using APS. For instance, Mishra et al. [75,76,77] have studied the corrosion resistance of APS nickel-based alloy coatings in actual boiler environments. The corrosion resistance mechanisms among the nickel-based coatings is the formation of protective oxides such as NiO, Cr2O3, NiCr3O4, and Y2O3, depending on the oxide-forming elements. In boiler environments, the protective oxides on the surface or along splat boundaries of the coatings blocked and retarded diffusion of corrosive mediums from the deposit to the substrates, resulting in prevention of corrosion. Although nickel-based alloy coatings are effective against high temperature corrosion in boiler environments, production cost may be high because the materials are expensive. Jiang et al. [78] reported that iron-based alloy containing Ni–Cr also formed similar protective oxides/spinels against corrosive alkali metals. This is less expensive alternative for corrosion resistance applications. The iron-based coatings also demonstrated high temperature corrosion resistance against KCl deposits.
Protective oxides such as NiO, Cr2O3, and NiCr3O4 formed from the elements in APS coatings play an important role in corrosion protection. Alloys containing nickel and chromium are oxidised to form protective oxides during the APS process, yet the formation of protective oxides could be further enhanced by post processing. Pre-oxidation is a heat treatment process to encourage APS coatings to further form protective oxides. The protective oxide scales are deliberately introduced to the materials by exposing the specimens at elevated temperature with controlled flow of oxygen [84]. Due to pre-oxidation, the formation of protective oxide scales provided enhanced high temperature alkali-induced corrosion resistance of boiler parts [85, 86]. Wu et al. [83] studied the effects of pre-oxidation of NiAl APS coatings on KCl corrosion resistance. Pre-oxidation process of the coatings was conducted at 700 °C in a static air atmosphere for 6 h. Protective layers of NiO were formed on the surface and splat boundaries of the NiAl coating after the pre-oxidation process, as demonstrated by a schematic diagram in Fig. 14. During KCl exposure at 600 °C, chloride ions (Cl−) reacted with metal elements of coatings and released Cl2 in the process. As sprayed NiAl suffered from the diffusion of Cl2 attacking the metal surface underneath the coating to form NiCl2, which was later oxidised to form non-protective NiO. On the other hand, the formation of protective NiO along the splat boundaries of pre-oxidised NiAl coating reduced porosity and delayed the diffusion of Cl2 gas. Cross-sectioned microstructures of as-sprayed and pre-oxidised NiAl coatings are shown in Fig. 15.
a Diffusion path of Cl2 in as-sprayed NiAl coating, and b formation of protective NiO layers along NiAl splat boundaries after pre-oxidation and altered Cl2 diffusion path, adapted and redrawn from [83]
Cross-sectioned microstructures and X-ray map** of a as-sprayed NiAl coating, and b pre-oxidised NiAl coating showing a thin oxide layer on the exposed surface and along splat boundaries [83]
Corrosion-Resistant Ceramic APS Coatings
Alumina (Al2O3) and titania (TiO2) are common ceramic materials for corrosion resistance that are applied by APS [79,80,81]. The ceramic coatings provided protection against high temperature corrosion by preventing corrosive species from deposit to reach the surface of substrate [82]. Interestingly, Kumar et al. [79] demonstrated the use of carbon nanotube (CNT) as a reinforcing material for zirconium–yttrium (ZrO2–Y2O3) APS coatings for high temperature corrosion resistance against molten Na2SO4–V2O5 for 50 cycles of 1-h cyclic exposure at 600 °C. The CNTs reduced coating porosity and eliminated diffusion paths for corrosive species from the deposits to reach the substrate. Therefore, the corrosion resistance was not only obtained from ceramic components, but also contributed from the dense microstructure due to the presence of reinforcing CNTs.
However, ceramic coatings suffer from thermal expansion coefficient mismatch, resulting in defects that served as a pathway for corrosive species to reach the metal surface underneath the coating [80]. To minimise such issues, bond-coats and/or gradient coatings have been used. In this regard, Mohammadi et al. [87] studied the corrosion resistance of APS gradient yttria-stabilised zirconia–alumina (YSZ-Al2O3) coatings on a gradient CoNiCrAlYSi bond-coat against molten Na2SO4-NaVO3. Figure 16 shows the surface and cross-sectional microstructure of the gradient coating. The aluminised layer was fabricated on the CoNiCrAlYSi bond coat. Above these bond coat layers, a gradient top-coat of YSZ-Al2O3 was deposited using APS. The presence of a high aluminium content in the bond-coat encouraged the formation of thermally grown oxide (TGO) of alumina. This TGO chemically bonded with the Al2O3 in the ceramic top-coat, which reduced thermal stress caused by thermal expansion and minimised the risk of top-coat spallation.
SEM images of a surface, and b cross-sectioned microstructure/architecture of gradient YSZ-Al2O3 top-coat on aluminised CoNiCrAlYSi bond coat [87]
High Velocity Oxygen Fuel (HVOF) Process
High velocity oxygen fuel (HVOF) process uses fuels such as acetylene, liquid kerosene, hydrogen, or propylene and an oxidising agent (oxygen) to generate intense heat to fully or partially melt feedstock powders and propel them onto a substrate using supersonic jet to form a coating [88]. A schematic diagram of HVOF process is shown in Fig. 17. HVOF is regarded as a standard method to deposit ceramic-metal (cermet) coatings [89]. HVOF is the most widely used process in the past decade to produce corrosion-resistant coatings for boiler applications as indicated by the number of published studies summarised in Table 4.
Schematic diagram of high velocity oxygen fuel (HVOF) process, adapted and redrawn from [90]
Corrosion-Resistant Alloy HVOF Coatings
Nickel-based alloys are among the most widely used materials for high temperature corrosion-resistant HVOF coatings, as summarised in Table 4. These coating materials contain alloying elements such as nickel, chromium, aluminium to form protective oxides and spinels against high temperature corrosion in boilers. Chatha et al. [96] studied the corrosion resistance of HVOF-sprayed NiCr coating in a coal-fired boiler. The coating remained intact after 1500 h of exposure. The corrosion resistance was reported to be attributed by two factors that prevented penetration of corrosive species through coating. These factors included (i) formation of protective NiO and Cr2O3 layers and (ii) a dense microstructure of splats parallel to the substrate. Moreover, nickel-based alloys with high chromium content have demonstrated excellent corrosion resistance in boilers. A HVOF Ni-46Cr coating formed a protective Cr2O3 layer and showed superior corrosion resistance after 1300 h of exposure in a biomass co-fired boiler comparison to coatings with lower chromium content [101]. Interestingly, iron-based HVOF Fe-27Cr coating could also produce sufficient protective oxides to withstand corrosion in a biomass-fired boiler for 2 years without diffusion of chlorine into the coating [99]. This could be a low-cost alternative to nickel-based alloys for corrosion-resistant coatings. The Cr2O3 is not the only protective phase against high temperature corrosion in boilers. Alloying element such as aluminium in nickel-based HVOF coatings also produces a protective layer of Al2O3 against corrosion attacks in both laboratory-scale and boiler environments [91, 104].
Apart from the formation of the protective oxides of HVOF coatings, coating microstructures also play an important role in providing protection against corrosion. Corrosion initiates from splat boundaries and spreads through the coating to the substrate [108]. Therefore, it is important for corrosion-resistant coatings to provide highly dense microstructures that prevent corrosive species from penetrating through the coatings. In this regard, Oksa et al. [98] have demonstrated that dense HVOF nickel-based coatings where interconnected pores were not observed in the coatings sustained two-year exposures in a biomass-fired boiler. Although dense microstructures are crucial for corrosion prevention, dense coatings alone may not provide sufficient protection if a continuous layer of protective oxides is absent. Bala et al. [92] reported that although cold-sprayed NiCr showed denser microstructure compared to the HVOF counterpart coating, yet HVOF NiCr coating preformed superior corrosion resistance in a boiler environment. Relatively low temperature of cold spray produced less amount of protective Cr2O3 compared to HVOF. Moreover, it was found that, unlike the HVOF NiCr coating, the Cr2O3 layer of cold spray NiCr was discontinuous, which attributed to an inferior corrosion resistance of cold spray NiCr, compared to the HVOF counterpart.
Corrosion-Resistant Cermet HVOF Coatings
Hard materials such as chromium carbide (Cr3C2), tungsten carbide (WC), boron carbide (B4C), and silicon carbide (SiC) have been used to form HVOF cermet coatings for corrosion resistance in boilers. Normally, hard materials such as Cr3C2 has been used with NiCr to improve the wear resistance of HVOF coatings [109, 110]. Nevertheless, several researchers have developed Cr3C2–NiCr coatings by HVOF and studied their high temperature corrosion behaviours in molten salts and boilers [93,94,95, 97, 102, 103]. Despite the addition of the carbide phase, the corrosion resistance mechanisms of HVOF Cr3C2–NiCr coatings are similar to those of their NiCr counterpart. High temperature corrosion resistance is attributed to the formation of protective oxides or spinels [94, 95, 97].
Zhou et al. [102] studied the corrosion resistance of HVOF Cr3C2–NiCr and Cr3C2–NiCrMoNbAl coatings by exposing them to molten NaCl–KCl–Na2SO4. As represented in Fig. 18, the carbide phase was evenly distributed throughout the dense metal matrix of the coatings, which is represented by the darker area in the microstructures. It was found that HVOF Cr3C2–NiCrMoNbAl coating provided superior corrosion resistance to the HVOF Cr3C2–NiCr counterpart. The thickness loss of these coatings after corrosion was approximately 70 μm and 200 μm out of almost 400 μm thickness of as-sprayed coatings, respectively. Corrosion mechanisms for these coatings are detailed in Fig. 19. Firstly, a simplified schematic diagram of both coating is presented in Fig. 19a. During high temperature corrosion, a protective layer of Cr2O3 and NiCr2O4 was formed on the surface of the Cr3C2–NiCr coating. The protective oxides for the Cr3C2–NiCrMoNbAl coating were more complex, which included Cr2O3, NiO, NiCr2O4, Al2O3, MoO, and NbO. Hard chromium carbide was dispersed across the whole coating. Secondly, Fig. 19b shows that the presence of chlorides could deplete the protective Cr2O3 coating and further caused active oxidation, as-explained in Sect. “High Temperature Corrosion by Slag Deposits on Boiler Steels”. Coating spallation occurred along the cracks formed during corrosion. On the contrary, protective oxides of the Cr3C2–NiCrMoNbAl coating were more complex and the presence of oxides apart from Cr2O3 enhanced the corrosion resistance. Finally, Fig. 19c indicates that (I) the presence of a carbide phase could alter the diffusion path of corrosive species and, thereby, enhance the corrosion resistance that could further improve the protection of Cr3C2–NiCrMoNbAl. However, (II) if Cl2 eventually penetrates the coating and reaches the coating–substrate interface, severe corrosion can occur and cause coating spallation.
Cross-sectioned microstructures of HVOF sprayed a Cr3C2–NiCr, and b Cr3C2–NiCrMoNbAl coatings [102]
Schematic representation of diffusion of corrosive species in carbide-based coatings, adapted and redrawn from [102]
Singh et al. [107] introduced SiC and B4C to NiCrAlY feedstock to fabricate HVOF coatings and investigated their effects on corrosion resistance against molten Na2SO4–V2O5. It was found that formation of protective oxides such as NiO, Cr2O3, and NiCr2O4 provided corrosion resistance. Each carbide material provided coating improvements. A 80 wt% NiCrAlY—20 wt% SiC coating provided superior corrosion resistance due to the formation of self-healing and a passive SiO2 scale. However, a 80 wt% NiCrAlY—20 wt% B4C produced fluxing B2O3 that facilitated crack restoration in the coatings.
Carbon nanotubes (CNTs) have been used as a reinforcing material, and also improved the corrosion resistance. Goyal et al. [105] pioneered the addition of 1–2 wt% CNTs as a reinforcing material to Cr3C2–20NiCr HVOF coatings and studied their corrosion resistance in an industrial boiler. The reinforcing CNT filled porosity of the coating matrix resulted in denser microstructures. Coating porosity without CNTs was 1.85%. Addition of 1 wt% and 2 wt% CNTs reduced coating porosity to 1.81% and 1.74%, respectively. Denser coating microstructures limited diffusion paths for corrosive species to penetrate the coating. The corrosion rates were reduced by 80.71% and 82.96% when 1 wt% CNTs and 2 wt% CNTs were added to the Cr3C2–20NiCr coating, respectively.
Detonation Gun (D-gun™) Coatings
Detonation gun (D-gun™) shares some similarities with HVOF except it relies on repetitive explosion of oxygen-fuel gas mixtures Fig. 20. A mixture of combustible gas and oxygen is injected to the chamber and powder is injected into the barrel. A spark plug ignites the gas mixture, generating heat and a high-pressure gas. Unlike HVOF, the explosion of oxy-fuel mixtures is not continuous combustion. This process allows gas temperatures 3700 to 4700 K and high velocity molten particles of 1400–1600 m/s to form highly and well-bonded coatings [111]. Nitrogen gas is then used to purge the barrel of residual combustion products before the next explosion cycle. The term and process "D-gun™" are proprietary [61]. However, non-propriety processes are available. Recent studies on D-gun™ coatings on corrosion resistance applications in boilers are summarised in Table 5.
Schematic diagram of detonation gun spraying process, adapted and redrawn from [111]
Corrosion-Resistant Alloy D-gun™ Coatings
As summarised in Table 5, corrosion resistance of D-gun™ coatings attributed by the presence of protective oxides and spinels. Nickel-based alloys with high content of chromium are widely used for corrosion-resistant coatings deposited by D-gun™. Kuashal et al. [115] detected the presence of a protective outermost Cr2O3 layer on the surface of D-gun™ NiCr coating and reported that the Cr2O3 layer blocked oxidising species from reaching the coated surface. A D-gun™ NiCr coating has demonstrated superior corrosion resistance in a molten Na2SO4–V2O5, compared to HVOF and cold spray counterparts [116]. It was reported that the protective Cr2O3 layer of a D-gun™ coating was continuous, unlike that of cold sprayed NiCr. Moreover, undesirable iron diffusion from the substrate to the coatings was observed in both HVOF and cold-sprayed NiCr coatings, but not in D-gun™ coatings. Kuashal et al. [113] have investigated that D-gun™ and HVOF sprayed NiCr coatings remained protective after 1500 h of exposure in a boiler environment. Nevertheless, the corrosion resistance in a laboratory-scale investigation using molten Na2SO4–V2O5 indicated that only HVOF could survive aggressive corrosion without spallation, unlike its D-gun™ spray counterpart. The reasons for oxide scale spallation of D-gun™ NiCr coatings in the molten salt environment remained unclear.
Another comparative high temperature corrosion study by Sundaresan et al. [127] was conducted on NiCoCrAlY coatings deposited by D-gun™ and APS and exposed to a molten Na2SO4–K2SO4–Fe2O3 environment. Both coatings formed spinel NiCr2O4 that provided protection against molten salt attack, but the corrosion resistance of D-gun™ coating was superior. The better corrosion resistance of D-gun™ NiCoCrAlY coating was attributed to a denser microstructure with 0.9% porosity compared to 2.1% porosity of the APS coating, see Fig. 21. The denser microstructure limited the diffusion path for corrosive species from the molten salt environment.
Cross-sectional microstructure of NiCoCrAlY coatings deposited by a APS, and b D-gun™, adapted and rescaled from [127]
Corrosion-Resistant Ceramic D-gun™ Coatings
Table 5 indicates that alloys and cermets are more common corrosion-resistant coatings. The only ceramic that has been exploited D-gun™ applications is Cr2O3–Al2O3 [118, 119]. These studies, and others, have evaluated the corrosion or oxidation performance of detonation sprayed coatings by obtaining the parabolic corrosion rate constant (Kp) values from cyclic high temperature corrosion or oxidation experiments. Specifically, the weight gain of coating samples is recorded after each corrosion or oxidation exposure at elevated temperature. Then, Kp can be calculated by determining the slope of the curve of (weight gain/surface area)2—vs the number of cycles, as shown in Fig. 22. This method is not limited to only sprayed coatings and Table 6 summarises Kp values of uncoated steels and detonation sprayed coatings, obtained with similar testing procedures.
Fitting linear curve of (weight gain in mg cm−2)2-number of cyclic oxidation of coated and uncoated alloys after 100 cyclic oxidation in air at 900 °C [128]
Rani et al. [118, 119] studied the corrosion behaviours of D-gun™ Cr2O3–Al2O3 coatings on T-22 boiler steel and 800H superalloy in molten Na2SO4–V2O5 at 900 °C. Coatings on both the substrates formed oxide scales of Al2O3 and Cr2O3 as protection against the molten salt mixture, resulting in intact coatings after 50 cycles of exposures. As a result, the weight gain of coated specimens after cyclic hot corrosion was less than the uncoated ones. The Kp values of coated T-22 and 800H specimens were 72.77 × 10–8 g2 cm−4 s−1 and 0.39 × 10–8 g2 cm−4 s−1, respectively. However, Kp of T-22 steel coated with the Cr2O3–Al2 was reduced to 0.06 × 10–8 g2 cm−4 s−1 and Kp of coated 800H superalloy was reduced to 0.24 × 10–8 g2 cm−4 s−1. Furthermore, Kp values of other uncoated and detonation sprayed ones obtained under similar testing protocols are summarised in Table 6.
Cold Spray Coatings
Figure 23 shows a schematic diagram of the cold spray process where pre-heated high-pressure gas is used to propel coating materials. Cold spray forms coatings at a temperature lower than the melting point of feedstocks and uses high kinetic energy from a supersonic gas jet that propels coating materials to the substrates at velocities of up to 1500 m s−1 [131]. The cold sprayed coatings are formed in the solid state due to relatively low process temperature. Hence the feedstock materials are normally ductile, but brittle materials can also be sprayed by incorporating a deformable matrix [132]. Corrosion-resistant nickel-based alloys and cermet materials have been sprayed and their corrosion resistance has been studied for boiler applications, as summarised in Table 7. The corrosion resistance of these coatings arises predominantly from the formation of protective oxide scales.
Schematic diagram of cold spray process, simplified and redrawn from [133]
Corrosion-Resistant Alloy Cold Spray Coatings
As summarised in Table 7, nickel-based alloys with high chromium contents are widely used as corrosion-resistant cold spray coatings and the corrosion prevention mechanisms are dominated by the formation of protective oxides. Bala et al. [134] have developed cold spray NiCr coating and investigated their corrosion behaviours in molten Na2SO4–V2O5. The as-sprayed coatings mainly consisted mainly of nickel and chromium phases, but protective oxides such as NiO and Cr2O3 were formed during exposure to the molten salt. These oxides provided protection against high temperature corrosion, resulting in an intact coating after 50 cycles of molten salt exposure. Cold-sprayed NiCr coating demonstrated a similar corrosion prevention mechanisms in a boiler [135]. A comparative study by Bala et al. [136] investigated corrosion–erosion resistance behaviours of cold-sprayed Ni-20Cr and Ni-50Cr coatings. The coatings were exposed in a coal-fired boiler for 1500 h. The cold sprayed Ni-20Cr and Ni-50Cr coatings reduced corrosion–erosion rate by 58% and 72%, respectively. The corrosion resistance of cold-sprayed Ni-50Cr was superior to a Ni-20Cr counterpart owing to the higher chromium content that enabled formation of protective oxides. The microhardness of the as-sprayed Ni-50Cr (469 Hv) was higher than the Ni-20Cr counterpart (221 Hv) due to higher chromium content that could form a hard Cr2O3 phase during high temperature exposure. The hardness of thermally sprayed NiCr coatings was known to arise by solid solution strengthening [142]. In addition, a chromium-rich second phase of a NiCr-based coating was formed during the thermal spray process [143] that could also contribute to the increased hardness. This resulted in better corrosion–erosion resistance of cold-sprayed Ni-50Cr over the Ni-20Cr coating.
Apart from micron-sized feedstocks, Kumar et al. [137] used nano-sized NiCr feedstock to produce cold-sprayed coatings and investigated their corrosion–erosion resistance in a boiler. The reduction of coating thickness loss by 100% was reported, compared to uncoated boiler steels in the tested environment. Formation of Cr2O3 scale was attributed to corrosion resistance of the nano-sized cold spray NiCr coatings. The authors reported that microhardness of the nano-NiCr coating by cold spray was 2.5 times harder than that of micron-sized counterpart due to its fine grain size and high density. Similar findings were also reported in a similar study investigating nano-sized NiCr cold spray coating, which suggested that nano-sized NiCr offered formation of high-density protective Cr2O3 layer [138].
Corrosion-Resistant Cermet Cold Spray Coatings
Despite cold spray’s low processing temperature, ceramics can be sprayed when mixed with a ductile metallic matrix [131]. Ceramics and metals have been cold sprayed to form cermet coatings for improved corrosion and erosion resistance. Singh et al. [139] found that NiCrTiCRe coatings exhibited maximum corrosion resistance in molten Na2SO4–V2O5 due to Cr2O3 formation, titanium compounds, and inert rhenium dispersion. Similarly, Bala et al. [139, 140] developed NiCrTiCRe, NiCrTiC, and NiCr coatings tested in boilers and indicated similar corrosion resistance. The presence of TiC and rhenium in NiCrTiCRe increased the microhardness from 179 Hv to 279 Hv, hence offering superior protection against corrosion–erosion.
Anti-slagging Coatings for Boilers
Slag deposition onto boiler components reduces their thermal efficiency. Methods to fabricate anti-slag coatings for industrial boilers include ceramic slurry spray and thermal spray. Coatings that were specifically designed for anti-slagging functionality have not received much attention compared to coatings designed for corrosion resistance.
Ceramic Slurry Spray Coatings to Prevent Slag
Ceramic and glass–ceramic coatings have been produced using a slurry spray method for applications such as environmental barriers [55], oxidation resistance [54], and boilers [144,145,146,147]. The raw materials are prepared as a ceramic slurry composed of binder materials, fine ceramic and metal powders, and other additives, as reported by Nguyen [148]. The ceramic slurry can then be spray deposited onto the substrates. The slurry spray process is explained in Sect. “Slurry Spray Coatings”.
For boiler applications, ceramic slurry coatings are applied on the heat exchanger surfaces to prevent or reduce unwanted slag deposition. The slag reduction by coatings is contributed by two mechanisms, which are (I) prevention of high temperature corrosion from slag deposits [144, 149, 150] and (II) reducing the wettability of molten fly ash on the coated surfaces [145, 147]. The slag reduction is achieved when the factors contributing to an increase in the slag adhesion are suppressed. Development of slurry spray coatings for anti-slagging functionality are summarised in Table 8.
The slurry spray process is inexpensive and simple for large-scale boilers but have not received much attention; possibly due to the long processing times that involve multiple steps of spraying, drying, curing, and post heat treatment.
Despite technical drawbacks, slurry spray is a simple and economical process to manufacture ceramic coatings [151] that has produced ceramic coatings for boiler applications. For example, Nguyen et al. [148] developed ceramic slurry spray coatings for high temperature and humid environments using a slurry of silicate binder materials and ceramic metallic powders. The coated samples exhibited improved oxidation resistance due to a dense and crack-free microstructure of the coating and the formation of a protective film on the steel substrate that reduced oxygen diffusion.
Nguyen et al. [144] also developed a slurry spray ceramic coating with an anti-fouling functionality for industrial boilers. The coating was composed of potassium silicate as a binder material and aluminium flakes and other ceramic oxides as filler materials. Fly ash firmly adhered to the uncoated specimen after high temperature exposure in a laboratory-scale test due to chemical interactions between the fly ash and the unprotected substrate. On the other hand, the coated sample prevented fly ash from adhering to the surface. The field test for the developed coating was executed in an industrial boiler for three months. The coating applied on the water wall of the boiler resulted in weakly bonded slag deposits that could be removed by using an air gun at low pressure.
Slag formation mechanisms are associated with an initial sticky layer of slag deposits that promotes further growth on the surface of boiler components [10]. The high surface energy of bare metal heat exchanger surfaces is prone to wetting by molten alkali salts from biomass combustion, which could serve as the foundation layer for further slag deposition. To prevent this issue, heat exchanger surfaces may be coated with low surface energy materials [152]. Hexagonal boron nitride (h-BN) is a low surface energy material that has been used as a component of ceramic composite coatings for anti-slagging purposes for boilers. Wang et al. [146, 147] investigated the lyophobicity and slag reduction by h-BN-graphite composite ceramic coating on iron-based 15CrMo alloy. The lyophobicity of such coatings was reflected by poor wettability of molten Na2SO4 droplets—an alkali salt to form the initial sticky layer—on the coated surface compared to uncoated metal. This was due to weaker interaction between Na–B or Na–N atoms, compared to Na–Fe atoms (i.e. the uncoated steel). The coated surface reduced the wettability of Na2SO4, resulting in a weakened ability of the initial sticky layer to capture fly ash particles and restrict the further formation of the slag deposits. Therefore, slag deposition was reduced and eventually, the slag fell off the coating, as demonstrated in Fig. 24.
Slag reduction mechanisms by a protective coating altering the wettability of incoming slag deposition [147]
Thermal Spray Coatings to Prevent Slag
Naganuma et al. [149] investigated the effects of arc sprayed nickel alloy coatings in reducing the adhesion strength of fly ash pellets under high temperature at a constant pressure. The adhesion strength of the fly ash on austenite stainless steel coated with an arc-sprayed nickel alloy was lesser compared to the uncoated one. The reduction of adhesion was attributed to the prevention of high temperature corrosion caused by the corrosive species in the fly ash pellet. The arc-sprayed nickel coatings prohibited the diffusion of iron from the substrate to the fly ash pellet and weakened the adhesion strength at the interface. The same nickel alloy coating was investigated for slag reduction in industrial boilers [150] and showed consistent results. The coatings prevented corrosion and reduced the amount of deposited slag on the heat exchanger surfaces after 1–2 years of exposure in industrial boilers, as shown in Fig. 25.
Photographs of slag deposition on boiler tubes with an arc spray nickel coating compared to uncoated area after 1 year (left), and 2 years of service (middle), and a schematic diagram of ash deposition on the coated and uncoated area of a boiler tube [150]
Methods to Evaluate Slag Resistance of Coatings for Boiler
There is no accepted standard test to evaluate the slag and fouling resistance of boiler surfaces or coatings. Evaluating the anti-slagging performance of coatings in operational boilers would be the most robust method. This approach, however, may take many months and interfere with plant production efficiencies. Thus, laboratory screening tests can evaluate the slag reduction capability that would justify the need for further industrial trials in operational boilers. Several studies [145, 149, 153,154,155] have investigated the adhesion behaviour of slag deposits on coated and uncoated surfaces. These studies include measurement of the shear strength and tensile strength of artificial slag deposits or contact angle measurements of molten ash.
Shear Adhesion Strength Measurement of Slag Deposits
Laxminarayan et al. [153] developed a robotic arm to measure the shear adhesion strength of slag deposits from a boiler and artificial fly ash deposits, as shown in Fig. 26. The deposits were formed into a cube shape using the Teflon mould and sintered onto a metal tube, which was followed by shear adhesion strength testing. The shear adhesion strength of the deposits was measured by a load cell on the robotic arm. The shear adhesion strength depended on factors such as sintering temperature, ash deposit compositions, and eutectic point of the ash deposits. The adhesion strength varied significantly, ranging from 1 to 350 kPa, depending on the aforementioned factors. This wide range of observed shear strength indicates the difficulty in obtaining comparable results when using different types of biomass ash or varying experimental conditions.
A 3D diagram of the setup to investigate the shear strength of artificial deposits on boiler tube [153]
Tensile Adhesion Strength Measurement of Slag Deposits
Naganuma et al. [149] studied slag reduction mechanisms of a nickel alloy arc sprayed coatings by measuring the tensile adhesion strength of fly ash pellets doped with alkali sulphates on nickel coated and uncoated specimens. The pellets and specimens were exposed to 1020 °C under 40 N pressure to firmly adhere the slag deposits to the substrate surface. After high temperature exposure, the load cell was slowly removed from the pellet and the tensile adhesion strength was calculated. Adhesion strength between the fly ash pellet and specimens increased over time. Uncoated steel exhibited the highest adhesion force of 1400 kPa even after exposure for approximately 140 h. Meanwhile, nickel-coated samples showed significantly reduced adhesion strength of below 600 kPa after 500 h of exposure. Another example of tensile strength measurement of the deposits on a boiler tube was performed by Laxminarayan et al. [154] using an electrically controlled arm to pull deposit samples from a superheater tube, as shown in Fig. 27. Ash deposits were placed on an air-cooled boiler tube and sintered in a furnace prior to tensile test. Strength measurement arms were used to slowly pull the ash deposits away from the air-cooled boiler tube at the rate of 2.5 mm s−1. Further, the tensile adhesion strength at the interface of the ash deposits and boiler tube was calculated by dividing the force required to separate ash deposit from the steel to the contacting area.
Testing apparatus used to measure tensile strength of ash deposits on an air-cooled superheater tube [154]
Contact Angle Measurement of Molten Deposits
Figure 2 shows that one of the slag formation mechanisms is deposition of molten fly ash that impacts the surface of boiler components. The wetting characteristics of the heat exchanger surfaces contribute to the adhesion of slag. Generally, high wettability of the boiler surface leads to a larger contact area at the liquid–solid interface between the boiler steel and deposited molten fly ash, which results in higher adhesion strength [145]. Therefore, the wettability of molten particles play an important role in the slag deposition. The contact angle at the interface between the liquid droplet and the solid surface represents the contact surface area at the liquid–solid boundary.
Wang et al. [145] evaluated the anti-slagging capability by measuring the contact angle of molten artificial ash droplets as displayed in Fig. 28. The ash was pre-melted at 1300 °C in the top furnace prior to transportation to a cooler furnace where the contact angle of a molten droplet was measured in a N2 atmosphere. The results showed that the use of low surface energy coatings such as h-BN reduces the contact angle of the molten ash in comparison with the surface of a bare steel. Thus, the poor wettability and reduced contact interface weakens the adhesion of the ash that accumulates on the boiler surface, and eventually, the slag will shed of its own accord owing to poor adhesion. A similar approach to measure the contact angle of coatings was conducted by Naganuma et al. [155] by using the soda lime glass as a molten droplet. The soda lime glass represents the deposits from combustion processes in boilers.
Experimental setup used to measure the contact angle of molten artificial ash on coatings [145]
New Generation Slag Reduction Coatings and evaluation Methods
Developments of anti-slagging coatings lag behind the advancement of corrosion-resistant coatings for boilers. As a result, attention for research and development on this type of coatings is needed. Moreover, as discussed in Sect. “Methods to Evaluate Slag Resistance of Coatings for Boiler”, although researchers have explored different methods to evaluate the anti-slagging capability of coatings, there is no standard procedure to evaluate this property. These research gaps can be addressed by proposing (I) anti-slag coatings for boilers by thermal spray methods and (II) a standard method to evaluate the anti-slagging capability of coatings; topics that will be addressed in this section.
Superhydrophobic Surfaces for Boilers
Conventional thermal spray processes use micron-sized powder feedstocks and consequently form coating structures on the micrometre scale. On the other hand, coatings produced by submicron sized powders generally result in more refined coating structures that exhibit enhanced mechanical [156, 157] and thermal performance [158, 159]. Nevertheless, the use of particles below 5 μm for thermal spray is challenging because the ultra-fine particles have inadequate inertia to enter the core of thermal spray flame. The introduction of liquid thermal spray (LTS) [160, 161] has circumvented this technical issue and enables sub-micron feedstocks to be thermal sprayed. The LTS allows the formation of microstructures that are unobtainable by traditional thermal spray processes and other coating technologies. For example, suspension plasma spray (SPS, a subset of LTS processes) can create thermal barrier coatings with unique columnar microstructures [159, 162, 163]. Another example is the manufacture of hierarchical microstructures, as shown in Fig. 29 where SPS of nano-sized feedstocks has been implimented [164].
Hierarchical microstructure of TiO2 coating prepared by suspension plasma spray process: a, b top view of the coating, and b, c cross-sectional microstructure of the coating [164]
These microstructures confer coating characteristics that are distinct from traditional thermal spray processes. For example, hierarchical structures can provide superhydrophobic functionality as illustrated in Fig. 30. The microstructures provide nano-sized asperities that form porosity, which prevent droplet penetration to the substrate, hence, reduces the contact area at the liquid–solid interface [165]. The superhydrophobic surfaces repel liquid and enable high mobility of liquid droplets.
Schematic diagram of liquid droplet wetting behaviours on different types of surface microstructures [165]
Moreover, SPS offers a less complex approach to produce coatings that may potentially alter the wettability of molten ash particles in boilers compared to similar coating prepared by plasma spray physical vapour deposition (PS-PVD) [166]. The PS-PVD coating exhibited an ability to reduce the wettability of molten ash at 1200 °C due to its cauliflower-like microstructure, which was similar to SPS coatings. Thus, SPS coatings with such a microstructure may also provide similar performance against molten ash in boilers at elevated temperature.
However, there are some disadvantages of SPS to consider. SPS generally requires a lesser spray distance compared to a conventional APS process. This limits the plasma spray torch movements for the substrates with a complex geometry [167]. Consequently, this may impose challenges to deposit coatings on the surface of the acute angled aspects of boiler tubes or between the tubes.
Superhydrophobic LTS coatings have the potential to impede wetting of molten fly ash on boiler components and mitigate the formation of slag deposits. As well, the superhydrophobic coatings may be applied onto corrosion-resistant coatings so that these multilayered coatings provide both the ability to resist corrosion and prevent slag deposition. Figure 31 proposes the novel thermal spray coating architecture that serves these dual functions. First: a dense NiCr alloy corrosion-resistant coating is deposited adjacent to the substrate. The key function of this layer, about 100—200 μm thick, is to form a protective oxide scale that blocks corrosive species from contacting the boiler substructure. Second: a superhydrophobic coating with hierarchical structure, prepared by LTS, forms the top coating. This layer of 20–50 μm thickness diminishes the wettability of molten ashes and prevents slag deposition. The LTS coating materials need to be compatible to the composition of the first thicker layer. For example, a LTS Cr2O3 top layer on either dense NiCr or dense FeCr inner layer coating. Whereas it has been proven that dense Cr2O3 coatings can be applied by SPS [168], it would be necessary to adjust the spray parameters to manufacture coatings with a hierarchical structure that exhibits tortuous porosity.
Flame Spray Deposition of Artificial Slag on Boiler Surfaces
A bespoke “slag testing rig” has been developed and prototyped to simulate slag deposition processes in boilers. A schematic of the fully functional slag testing rig is shown in Fig. 32. This custom high temperature apparatus mimics the slag deposition process in boilers by employing a flame spray torch and water-cooled sampling probe. The thermal spray torch is fuelled with acetylene (C2H2) and oxygen as a heat source to melt biomass fly ash and propel the molten ash droplets to a testing surface clamped on a water-cooled probe. This test replicates similar conditions to those of molten fly ash particles impacting on heat exchanger tubes of boilers. A thermocouple monitors the specimen temperature so that cooling water can be adjusted to a specific surface temperature of the specimen.
Procedurally, fly ash is thermally sprayed onto coated coupons. Each coated coupon is subjected to high temperature exposure in an oxidising atmosphere to allow chemical reactions between the thermally sprayed fly ash and coating. This process simulates the high temperature corrosion that occurs on the boiler tube surface. High temperature corrosion by the ash deposits could contribute to the adhesion strength of the slag [149, 150]. Therefore, the coupon sprayed with fly ash should be initially exposed in a high temperature environment to allow any corrosion reactions. Finally, a tensile strength test on the fly ash layer investigates the adhesion of the deposited fly ash, which indicates the anti-slagging capability of the exploratory coated coupon.
Conclusions
Two protective coatings used in industrial boilers include (I) corrosion-resistant coatings and (II) anti-slagging coatings. Their properties and future prospects are summarised below.
Corrosion-resistant coatings: Thermal spray coatings to resist high temperature corrosion in boilers have been widely investigated. Nickel-based alloys with high chromium content are the most used materials to prevent corrosion and are effective in industrial boiler environments. The corrosion resistance is due to the formation of protective oxides of nickel and chromium and their spinels regardless of the specific thermal spray process. However, NiCr alloys may not be the most economical solution for anti-corrosion coatings and less expensive FeCr alloys are receiving research attention.
Oxide ceramic coatings have shown promising corrosion resistance against molten salts in laboratory experiments. However, there is still need to evaluate the corrosion resistance of ceramic coatings in functioning boilers. In addition, thermal mismatch of a ceramic coating on metallic boiler components is another challenge to be addressed.
In terms of advancement of coating materials, an interesting aspect of the corrosion-resistant coatings is the use of nano-sized raw materials. Nevertheless, only a handful of studies focused on the use of nano-feedstocks for corrosion-resistant coatings despite some benefits over micron-sized counterparts. As well, reinforcing materials were added to the matrix of coatings to further improve their corrosion resistance. Hard ceramics such as chromium carbide were introduced as a core material to metal wires for arc spray. This allowed alloy-carbide coating deposition for boiler tubes using arc spray, which was previously limited to the use of electrically conductive wires only. Carbon nanotubes (CNTs) are another material being introduced to thermal spray coatings as a reinforcing component to further enhance corrosion resistance. It was promising that these materials added to corrosion-resistant materials could improve the performance of the coatings against corrosion attack.
Anti-slagging coatings: The research progress on anti-slagging coatings is behind that of corrosion-resistant coatings although slag deposition is a significant issue for boilers. The commonly observed slag reduction mechanisms using coatings include (I) prevention of corrosion on boiler steels since this also contributes to slag adhesion, and (II) weakened adhesion and wettability of slag with a low surface energy material, specifically hexagonal boron nitride. However, there are two major research gaps of slag resistance coatings. Firstly, slurry spraying is the current primary method to deposit ceramic coatings with anti-slagging functionality, but this process is time consuming. Unlike ceramic counterparts, alloy coatings have already been deployed to reduce slag in boilers using arc spray, which is a faster coating production process. This creates a research gap for future development of ceramic coatings to reduce slag deposition in boilers using more rapid coating deposition techniques such as thermal spray processes. Secondly, although research groups have appraised the adhesion behaviour of slag on boiler steels or coatings, there is still lack of standardised methods to artificially deposit the slag on solid surfaces or coatings and evaluate their anti-slagging capability. A potential thermal spray coating architecture has been proposed to prevent slag deposition by harnessing superhydrophobic functionality without compromising high temperature corrosion resistance. In addition, for further anti-slagging investigation of coatings, a testing device has been employed to artificially deposit slag on water-cooled coating coupons using the flame spray technique so that a slag-forming environment in boilers can be simulated.
References
Y. Niu, H. Tan, and Se. Hui, Progress in Energy and Combustion Science 52, 1 (2016). https://doi.org/10.1016/j.pecs.2015.09.003.
J. Tharajak and N. Sanpo, Applied Mechanics and Materials 891, 137 (2019). https://doi.org/10.4028/www.scientific.net/AMM.891.137.
K. Khantisopon, J. Tharajak, P. Nawarat, N. Sanpo, and A. S. M. Ang, Corrosion Science 205, 110470 (2022). https://doi.org/10.1016/j.corsci.2022.110470.
Reducing costs through improving boiler maintenance efficiency. Milling Matters, 2018. Sugar Research Australia Limited. https://elibrary.sugarresearch.com.au/handle/11079/17318
K. Szymański, A. Hernas, G. Moskal, and H. Myalska, Surface and Coatings Technology 268, 153 (2015). https://doi.org/10.1016/j.surfcoat.2014.10.046.
Y. Zhang, Q. Li, and H. Zhou, Chapter 6—Effects of Ash Deposition and Slagging on Heat Transfer. in Theory and Calculation of Heat Transfer in Furnaces, eds. Y. Zhang, Q. Li and H. Zhou (Academic Press, Oxford, 2016), p. 173.
M. Broström, S. Enestam, R. Backman, and K. Mäkelä, Fuel Processing Technology 105, 142 (2013 ). https://doi.org/10.1016/j.fuproc.2011.08.006.
X. **, J. Ye, L. Deng, and D. Che, Energy & Fuels 31, 2951 (2017). https://doi.org/10.1021/acs.energyfuels.6b03381.
H. Zhang, C. Yu, and Z. Luo, RSC Advances 10, 21420 (2020). https://doi.org/10.1039/D0RA04370B.
Y. Niu, Y. Zhu, H. Tan, S. Hui, Z. **g, and W. Xu, Fuel Processing Technology 128, 499 (2014). https://doi.org/10.1016/j.fuproc.2014.07.038.
M. Zhai, X. Li, D. Yang, Z. Ma, and P. Dong, Journal of Cleaner Production 336, 130361 (2022). https://doi.org/10.1016/j.jclepro.2022.130361.
L. Krumm and M. Galetz, Materials and Corrosion 71, 166 (2019). https://doi.org/10.1002/maco.201911091.
C. Berlanga and J. A. Ruiz, Journal of Chemistry 2013, 272090 (2013). https://doi.org/10.1155/2013/272090.
H. J. Grabke, E. Reese, and M. Spiegel, Corrosion Science 37, 1023 (1995). https://doi.org/10.1016/0010-938X(95)00011-8.
J. Pettersson, H. Asteman, J. E. Svensson, and L. G. Johansson, Oxidation of Metals 64, 23 (2005). https://doi.org/10.1007/s11085-005-5704-3.
J. Phother-Simon, T. Jonsson, and J. Liske, Corrosion Science 167, 108511 (2020). https://doi.org/10.1016/j.corsci.2020.108511.
S. C. Kung, Corrosion 71, 483 (2014). https://doi.org/10.5006/1409.
J. Pettersson, N. Folkeson, L.-G. Johansson, and J.-E. Svensson, Oxidation of Metals 76, 93 (2011). https://doi.org/10.1007/s11085-011-9240-z.
G. H. Meier, Oxidation of Metals 98, 1 (2022). https://doi.org/10.1007/s11085-020-10015-6.
J. J. Rowe, G. W. Morey, and C. S. Zen, Geological Survey Professional Paper 741, 1 (1972).
N. Abu-warda, A. J. López, F. Pedraza, and M. V. Utrilla, Corrosion Science 173, 108747 (2020). https://doi.org/10.1016/j.corsci.2020.108747.
A. Mlonka-Mędrala, K. Gołombek, P. Buk, E. Cieślik, and W. Nowak, Energy 188, 116062 (2019). https://doi.org/10.1016/j.energy.2019.116062.
J. H. Park, et al., Fuel 236, 792 (2019). https://doi.org/10.1016/j.fuel.2018.09.020.
L. Wang, G. Skjevrak, J. E. Hustad, and M. G. Grønli, Energy & Fuels 25, 5775 (2011). https://doi.org/10.1021/ef2007722.
S. Karlsson, L.-E. Åmand, and J. Liske, Fuel 139, 482 (2015). https://doi.org/10.1016/j.fuel.2014.09.007.
A. Talus, Y. Alipour, R. Norling, and P. Henderson, Materials and Corrosion 67, 683 (2016). https://doi.org/10.1002/maco.201508693.
Y. Zhu, Y. Niu, H. Tan, and X. Wang, Frontiers in Energy Research 2, 7 (2014). https://doi.org/10.3389/fenrg.2014.00007.
Y. Kawahara, Coatings 6, 34 (2016). https://doi.org/10.3390/coatings6030034.
J. R. Davis, 5.6 Weld Overlays. in ASM Specialty Handbook—Stainless Steels, (ASM International).
I. Hulka, et al., Materials 14, 5583 (2021).
L. N. Nazharova, G. G. Mingazova, and R. S. Saifullin, IOP Conference Series: Materials Science and Engineering 450, 032003 (2018). https://doi.org/10.1088/1757-899x/450/3/032003.
K. **ao, et al., Ceramics International 44, 11180 (2018). https://doi.org/10.1016/j.ceramint.2018.03.147.
C. Leyens and E. Beyer, 8—Innovations in laser cladding and direct laser metal deposition. in Laser Surface Engineering, eds. J. Lawrence and D. G. Waugh (Woodhead Publishing, 2015), p. 181.
M. A. Uusitalo, P. M. J. Vuoristo, and T. A. Mäntylä, Corrosion Science 46, 1311 (2004). https://doi.org/10.1016/j.corsci.2003.09.026.
X.-Z. Li, Z.-D. Liu, H.-C. Li, Y.-T. Wang, and B. Li, Surface and Coatings Technology 232, 627 (2013). https://doi.org/10.1016/j.surfcoat.2013.06.048.
L. Li, F. Shen, Y. Zhou, and W. Tao, Journal of Laser Applications 31, 042009 (2019). https://doi.org/10.2351/1.5094378.
T. Schopphoven, A. Gasser, and G. Backes, Laser Technik Journal 14, 26 (2017). https://doi.org/10.1002/latj.201700020.
T. Ge, L. Chen, P. Gu, X. Ren, and X. Chen, Optics & Laser Technology 150, 107919 (2022). https://doi.org/10.1016/j.optlastec.2022.107919.
F. Shen, W. Tao, L. Li, Y. Zhou, W. Wang, and S. Wang, Applied Surface Science 517, 146085 (2020). https://doi.org/10.1016/j.apsusc.2020.146085.
R. Wang, C. Ouyang, Q. Li, Q. Bai, C. Zhao, and Y. Liu, Materials 15, 1643 (2022). https://doi.org/10.3390/ma15051643.
X. Xu, et al., Surface and Coatings Technology 422, 127500 (2021). https://doi.org/10.1016/j.surfcoat.2021.127500.
W. Yuan, R. Li, Z. Chen, J. Gu, and Y. Tian, Surface and Coatings Technology 405, 126582 (2021). https://doi.org/10.1016/j.surfcoat.2020.126582.
J. P. Hurley, 15—High Temperature Heat Exchangers in Indirectly Fired Combined Cycle (IFCC) Systems: Materials Management and Performance Improvement. in Power Plant Life Management and Performance Improvement, ed. J. E. Oakey (Woodhead Publishing, 2011), p. 575.
L. Chen, et al., Corrosion Science 201, 110271 (2022). https://doi.org/10.1016/j.corsci.2022.110271.
C. C. Silva, Weld Overlay. in Encyclopedia of Tribology, eds. Q. J. Wang and Y.-W. Chung (Springer US, Boston, MA, 2013), p. 4094.
P. Petrzak, M. Blicharski, S. Dymek, and M. Solecka, Solid State Phenomena 231, 113 (2015). https://doi.org/10.4028/www.scientific.net/SSP.231.113.
M. Solecka, J. Kusinski, A. Kopia, M. Rozmus-Górnikowska, and A. Radziszewska, Acta Physica Polonica A 130, 1045 (2016). https://doi.org/10.12693/APhysPolA.130.1045.
M. Solecka, P. Petrzak, and A. Radziszewska, Solid State Phenomena 231, 119 (2015). https://doi.org/10.4028/www.scientific.net/SSP.231.119.
J. R. Nicholls, K. A. Long, and N. J. Simms, 4.05—Diffusion Coatings. in Shreir’s Corrosion, eds. B. Cottis, et al. (Elsevier, Oxford, 2010), p. 2532.
J. L. Dossett, and G. E. Totten, Part VIII. Diffusion Coatings. in ASM Handbook, Volume 04A—Steel heat Treating Fundamentals and Processes, eds. J. Dossett and G. E. Totten. (ASM International, 2013)
ASM Committee, 73.1.1 Principles of Pack Diffusion Coating. in ASM Handbook, Volume 05—Surface Engineering, eds. C. M. Cotell, J.A. Sprague and F. A. Smidt, Jr. (ASM International, 1994)
J. Metsäjoki, E. Huttunen-Saarivirta, and T. Lepistö, High Temperature Materials and Processes 30, 181 (2011). https://doi.org/10.1515/htmp.2011.027.
J. Metsäjoki, E. Huttunen-Saarivirta, and T. Lepistö, Fuel 133, 173 (2014). https://doi.org/10.1016/j.fuel.2014.05.017.
H. B. Hu, Y. Jia, Z. Q. Fang, and H. M. Zhou, Advanced Materials Research 779–780, 47 (2013). https://doi.org/10.4028/www.scientific.net/AMR.779-780.47.
R. Verma, S. Kant, and N. M. Suri, Proceedings of the Institution of Mechanical Engineers, Part E: Journal of Process Mechanical Engineering 230, 87 (2015). https://doi.org/10.1177/0954408915595948.
L. D. Bonifacio, B. V. Lotsch, and G. A. Ozin, 5.04—Periodic Mesoporous Materials: Holes Filled with Opportunities. in Comprehensive Nanoscience and Technology, eds. D. L. Andrews, G. D. Scholes and G. P. Wiederrecht (Academic Press, Amsterdam, 2011), p. 69.
J. Wang, Y. Yuan, Z. Chi, and G. Zhang, Materials Chemistry and Physics 206, 186 (2018). https://doi.org/10.1016/j.matchemphys.2017.12.025.
P. Nguyen, S. Harding, and S. -Y. Ho, Experimental studies on slurry based thermal barrier coatings. in Proceedings of the 5th Australasian Congress on Applied Mechanics, (Engineers Australia, Brisbane, Qld, 2007), p. 545.
W. F. Gale and T. C. Totemeier, 35.2 Chemical Vapour Deposition. in Smithells Metals Reference Book (8th Edition), (Elsevier. 2004).
S. Wijewardane, 10—Thermal spray coatings in renewable energy applications. in Future Development of Thermal Spray Coatings, ed. N. Espallargas (Woodhead Publishing, Sawston, UK, 2015), p. 241.
M. R. Dorfman, Chapter 22—Thermal Spray Coatings. in Handbook of Environmental Degradation of Materials (Third Edition), ed. M. Kutz (William Andrew Publishing, 2018), p. 469.
S. V. Joshi, D. Srinivasa Rao, and S. Centre for, N.-A. Technology of, and C. Other Develo**, Surface Engineering, (Daya Publishing House, New Delhi, India, 2010). (in English).
P. L. Fauchais, J. V. R. Heberlein, and M. I. Boulos, Wire Arc Spraying. in Thermal Spray Fundamentals: From Powder to Part, eds. P. L. Fauchais, J. V. R. Heberlein and M. I. Boulos (Springer, US, Boston, MA, 2014), p. 577.
V. N. Shukla, N. Rana, R. Jayaganthan, and V. K. Tewari, Procedia Engineering 75, 113 (2014). https://doi.org/10.1016/j.proeng.2013.11.025.
E. Qin, S. Yin, H. Ji, Q. Huang, Z. Liu, and S. Wu, Journal of Thermal Spray Technology 26, 787 (2017). https://doi.org/10.1007/s11666-017-0549-2.
J. Cheng, Y. Wu, L. Chen, S. Hong, L. Qiao, and Z. Wei, Journal of Thermal Spray Technology 28, 1263 (2019). https://doi.org/10.1007/s11666-019-00890-0.
X. Wang, D. He, X. Guo, Z. Zhou, G. Wang, and F. Guo, Surface and Coatings Technology 367, 173 (2019). https://doi.org/10.1016/j.surfcoat.2019.03.058.
S. Kumar, M. Kumar, and A. Handa, Materials Research Express 6, 125533 (2019). https://doi.org/10.1088/2053-1591/ab5fae.
S. Kumar, M. Kumar, and A. Handa, Materials at High Temperatures 37, 370 (2020). https://doi.org/10.1080/09603409.2020.1810922.
J. Cheng, Y. Wu, W. Shen, S. Hong, L. Qiao, and Y. Wang, Surface and Coatings Technology 397, 126015 (2020). https://doi.org/10.1016/j.surfcoat.2020.126015.
K. J. Stein, B. S. Schorr, and A. R. Marder, Wear 224, 153 (1999).
C. V. Maria, Sprays: Types, Technology and Modeling (Engineering Tools, Techniques and Tables), (Nova Science Publishers, Inc, New York, 2011). in English.
M. S. Sahith, G. Giridhara, and R. S. Kumar, Materials Today: Proceedings 5, 2018 (2746). https://doi.org/10.1016/j.matpr.2018.01.060.
B. A. Kahl, C. C. Berndt, and A. S. M. Ang, Surface and Coatings Technology 416, 127128 (2021). https://doi.org/10.1016/j.surfcoat.2021.127128.
S. B. Mishra, K. Chandra, and S. Prakash, Surface and Coatings Technology 216, 23 (2013). https://doi.org/10.1016/j.surfcoat.2012.09.044.
S. B. Mishra and S. Prakash, Surface Engineering 31, 29 (2015). https://doi.org/10.1179/1743294414Y.0000000338.
S. Mishra, K. Chandra, and S. Prakash, Anti-Corrosion Methods and Materials 64, 540 (2017). https://doi.org/10.1108/ACMM-11-2015-1592.
C. Jiang, et al., Surface Engineering 34, 634 (2018). https://doi.org/10.1080/02670844.2017.1319647.
S. Kumar, R. Bhatia, and H. Singh, Journal of Bio- and Tribo-Corrosion 6, 81 (2020). https://doi.org/10.1007/s40735-020-00378-3.
A. A. Mohammed, Z. T. Khodair, and A. A. Khadom, Ceramics International 46, 26945 (2020). https://doi.org/10.1016/j.ceramint.2020.07.172.
G. Singh, S. Kumar, and R. Kumar, Materials Research Express 7, 026527 (2020). https://doi.org/10.1088/2053-1591/ab6e7e.
M. S. Ansari, A. Bansal, V. Chawla, and V. Aggarwal, Surface Topography: Metrology and Properties 9, 025029 (2021). https://doi.org/10.1088/2051-672x/ac0171.
D. Wu, S. Liu, Z. Yuan, P. Cao, X. Wei, and C. Zhang, Journal of Thermal Spray Technology 2021. https://doi.org/10.1007/s11666-021-01248-1.
J. Lehmusto, D. Lindberg, P. Yrjas, and L. Hupa, Oxidation of Metals 89, 251 (2018). https://doi.org/10.1007/s11085-017-9785-6.
J. Lehmusto, P. Yrjas, and L. Hupa, Oxidation of Metals 91, 311 (2019). https://doi.org/10.1007/s11085-019-09898-x.
S. C. Okoro, M. Kvisgaard, M. Montgomery, F. J. Frandsen, and K. Pantleon, Surface Engineering 33, 428 (2017). https://doi.org/10.1080/02670844.2016.1199128.
M. Mohammadi, A. Kobayashi, S. Javadpour, and S. A. J. Jahromi, Vacuum 167, 547 (2019). https://doi.org/10.1016/j.vacuum.2018.04.042.
M. S. El-Eskandarany, 12—Utilization of Ball-Milled Powders for Surface Protective Coating. in Mechanical Alloying (Third Edition), ed. M. S. El-Eskandarany (William Andrew Publishing, 2020), p. 309.
N. Espallargas, 1—Introduction to Thermal Spray Coatings. in Future Development of Thermal Spray Coatings, ed. N. Espallargas (Woodhead Publishing, 2015), p. 1.
I. A. Alnaser, M. Yunus, R. Alfattani, and T. Alamro, Materials 14, 5119 (2021). https://doi.org/10.3390/ma14185119.
R. A. Mahesh, R. Jayaganthan, and S. Prakash, Surface Engineering 26, 413 (2010). https://doi.org/10.1179/174329409X451164.
N. Bala, H. Singh, S. Prakash, and J. Karthikeyan, Journal of Thermal Spray Technology 21, 144 (2012). https://doi.org/10.1007/s11666-011-9698-x.
S. S. Chatha, H. S. Sidhu, and B. S. Sidhu, Surface and Coatings Technology 206, 4212 (2012). https://doi.org/10.1016/j.surfcoat.2012.04.026.
S. S. Chatha, H. S. Sidhu, and B. S. Sidhu, Surface and Coatings Technology 206, 3839 (2012). https://doi.org/10.1016/j.surfcoat.2012.01.060.
M. Kaur, H. Singh, and S. Prakash, Metallurgical and Materials Transactions A 43, 2979 (2012). https://doi.org/10.1007/s11661-012-1118-4.
S. S. Chatha, H. S. Sidhu, and B. S. Sidhu, Journal of Thermal Spray Technology 22, 838 (2013). https://doi.org/10.1007/s11666-013-9899-6.
R. Bhatia, H. Singh, and B. S. Sidhu, Journal of Materials Engineering and Performance 23, 493 (2014). https://doi.org/10.1007/s11665-013-0771-0.
M. Oksa, P. Auerkari, J. Salonen, and T. Varis, Fuel Processing Technology 125, 236 (2014). https://doi.org/10.1016/j.fuproc.2014.04.006.
M. Oksa, T. Varis, and K. Ruusuvuori, Surface and Coatings Technology 251, 191 (2014). https://doi.org/10.1016/j.surfcoat.2014.04.025.
M. Oksa, J. Metsäjoki, and J. Kärki, Journal of Thermal Spray Technology 24, 194 (2015). https://doi.org/10.1007/s11666-014-0155-5.
M. Oksa, J. Metsäjoki, and J. Kärki, Coatings 6, 65 (2016).
W. Zhou, K. Zhou, C. Deng, K. Zeng, and Y. Li, Surface and Coatings Technology 309, 849 (2017). https://doi.org/10.1016/j.surfcoat.2016.10.076.
W. Zhou, K. Zhou, C. Deng, K. Zeng, and Y. Li, Ceramics International 43, 9390 (2017). https://doi.org/10.1016/j.ceramint.2017.04.109.
M. Bai, L. Reddy, and T. Hussain, Corrosion Science 135, 147 (2018). https://doi.org/10.1016/j.corsci.2018.02.047.
K. Goyal and R. Goyal, Surface Engineering 36, 1200 (2020). https://doi.org/10.1080/02670844.2019.1662645.
V. P. S. Sidhu, K. Goyal, and R. Goyal, Australian Journal of Mechanical Engineering 17, 127 (2019). https://doi.org/10.1080/14484846.2017.1364834.
G. Singh, N. Bala, V. Chawla, and Y. K. Singla, Corrosion Science 190, 109666 (2021). https://doi.org/10.1016/j.corsci.2021.109666.
S. Paul and M. D. F. Harvey, Journal of Thermal Spray Technology 22, 316 (2013). https://doi.org/10.1007/s11666-012-9820-8.
H. Singh and B. S. Sidhu, Materials Science Forum 751, 71 (2013). https://doi.org/10.4028/www.scientific.net/MSF.751.71.
S. Tailor, N. Vashishtha, A. Modi, and S. C. Modi, Materials Research Express 6, 076435 (2019). https://doi.org/10.1088/2053-1591/ab1947.
G. Sundararajan, D. S. Rao, G. Sivakumar, and S. V. Joshi, Detonation Spray Coatings. in Encyclopedia of Tribology, eds. Q. J. Wang and Y.-W. Chung (Springer US, Boston, MA, 2013), p. 736.
M. Kaur, H. Singh, and S. Prakash, Surface Engineering 26, 428 (2010). https://doi.org/10.1179/026708409X12490360425963.
G. Kaushal, H. Singh, and S. Prakash, Oxidation of Metals 76, 169 (2011). https://doi.org/10.1007/s11085-011-9245-7.
G. Kaushal, H. Singh, and S. Prakash, Materials at High Temperatures 28, 1 (2011). https://doi.org/10.3184/096034011X12960473417949.
G. Kaushal, H. Singh, and S. Prakash, Journal of Thermal Spray Technology 21, 975 (2012). https://doi.org/10.1007/s11666-012-9770-1.
G. Kaushal, N. Bala, N. Kaur, H. Singh, and S. Prakash, Metallurgical and Materials Transactions A 45, 395 (2014). https://doi.org/10.1007/s11661-013-1984-4.
A. Goyal, R. Singh, and G. Singh, Materials Today: Proceedings 4, 142 (2017). https://doi.org/10.1016/j.matpr.2017.01.007.
A. Rani, N. Bala, and C. Gupta, Anti-Corrosion Methods and Materials 64, 515 (2017).
A. Rani, N. Bala, and C. M. Gupta, Oxidation of Metals 88, 621 (2017). https://doi.org/10.1007/s11085-017-9759-8.
V. N. Shukla, H. Trivedi, H. Kumar, and A. Yadav, Materials Today: Proceedings 4, 10212 (2017). https://doi.org/10.1016/j.matpr.2017.06.350.
H. Gada, D. Mudgal, and B. Ahmad, Journal of Physics: Conference Series 1240, 012129 (2019). https://doi.org/10.1088/1742-6596/1240/1/012129.
R. Mittal, M. Singh, and P. Kumar, Materials Today: Proceedings 18, 4952 (2019). https://doi.org/10.1016/j.matpr.2019.07.487.
V. Singh, K. Goyal, and R. Goyal, Anti-Corrosion Methods and Materials 66, 394 (2019). https://doi.org/10.1108/ACMM-10-2018-2009.
H. Gada, D. Mudgal, S. Parvez, and B. Ahmad, Engineering Failure Analysis 108, 104256 (2020). https://doi.org/10.1016/j.engfailanal.2019.104256.
R. Mittal and H. Singh, Materials Today: Proceedings 26, 549 (2020). https://doi.org/10.1016/j.matpr.2019.12.136.
V. Saroop, D. Mudgal, S. Kumar, and S. Prakash, Engineering Failure Analysis 118, 104905 (2020). https://doi.org/10.1016/j.engfailanal.2020.104905.
C. Sundaresan, B. Rajasekaran, S. Govindarajan, and D. Rao, IOP Conference Series: Materials Science and Engineering 872, 012092 (2020). https://doi.org/10.1088/1757-899X/872/1/012092.
S. Kamal, R. Jayaganthan, and S. Prakash, Journal of Alloys and Compounds 472, 378 (2009). https://doi.org/10.1016/j.jallcom.2008.04.087.
S. Saladi, J. Menghani, and S. Prakash, Journal of Materials Engineering and Performance 24, 1379 (2015). https://doi.org/10.1007/s11665-015-1400-x.
S. Saladi, J. Menghani, and S. Prakash, Journal of Materials Engineering and Performance 23, 4394 (2014). https://doi.org/10.1007/s11665-014-1240-0.
B. AlMangour, Fundamentals of Cold Spray Processing: Evolution and Future Perspectives. in Cold-Spray Coatings: Recent Trends and Future perspectives, ed. P. Cavaliere (Springer International Publishing, Cham, 2018), p. 3.
A. Moridi, S. M. Hassani-Gangaraj, M. Guagliano, and M. Dao, Surface Engineering 30, 369 (2014). https://doi.org/10.1179/1743294414Y.0000000270.
R. Huang and H. Fukanuma, 6—Future trends in cold spray techniques. in Future Development of Thermal Spray Coatings, ed. N. Espallargas (Woodhead Publishing, 2015), p. 143.
N. Bala, H. Singh, and S. Prakash, Journal of Thermal Spray Technology 19, 110 (2010). https://doi.org/10.1007/s11666-009-9437-8.
N. Bala, H. Singh, and S. Prakash, Materials & Design 31, 244 (2010). https://doi.org/10.1016/j.matdes.2009.06.033.
N. Bala, H. Singh, J. Karthikeyan, and S. Prakash, Materials and Corrosion 64, 783 (2013). https://doi.org/10.1002/maco.201106387.
M. Kumar, H. Singh, N. Singh, and R. S. Joshi, Wear 332–333, 1035 (2015). https://doi.org/10.1016/j.wear.2015.01.043.
M. Kumar, H. Singh, N. Singh, N. M. Chavan, S. Kumar, and S. V. Joshi, Journal of Thermal Spray Technology 24, 1441 (2015). https://doi.org/10.1007/s11666-015-0249-8.
H. Singh, N. Bala, N. Kaur, S. K. Sharma, D. Y. Kim, and S. Prakash, Surface and Coatings Technology 280, 50 (2015). https://doi.org/10.1016/j.surfcoat.2015.08.002.
N. Bala, H. Singh, and S. Prakash, Surface and Coatings Technology 318, 50 (2017). https://doi.org/10.1016/j.surfcoat.2016.11.075.
H. Singh, S. S. Kalsi, and T. S. Sidhu, Materials and Corrosion 71, 232 (2020). https://doi.org/10.1002/maco.201911116.
D. Srinivasan, V. Chandrasekhar, R. Amuthan, Y. C. Lau, and E. Calla, Journal of Thermal Spray Technology 25, 725 (2016). https://doi.org/10.1007/s11666-016-0382-z.
S. Matthews and M. Schweizer, Journal of Thermal Spray Technology 22, 932 (2013). https://doi.org/10.1007/s11666-013-9939-2.
M. D. Nguyen, et al., Coatings 8, 353 (2018). https://doi.org/10.3390/coatings8100353.
G. Wang, et al., IOP Conference Series: Materials Science and Engineering 721, 012023 (2020). https://doi.org/10.1088/1757-899x/721/1/012023.
J. Wang, Y. Yuan, Z. Chi, and G. Zhang, Journal of the Energy Institute 91, 962 (2018). https://doi.org/10.1016/j.joei.2017.08.003.
J. Wang, G. Zhang, F. Li, and Z. Chi, Fuel Processing Technology 188, 43 (2019). https://doi.org/10.1016/j.fuproc.2019.02.012.
M. D. Nguyen, et al., Ceramics International 44, 8306 (2018). https://doi.org/10.1016/j.ceramint.2018.02.017.
H. Naganuma, et al., Fuel 106, 303 (2013). https://doi.org/10.1016/j.fuel.2012.11.017.
H. Naganuma, et al., Fuel Processing Technology 105, 77 (2013). https://doi.org/10.1016/j.fuproc.2011.09.009.
P. Nguyen, S.-Y. Ho, and A. Kotousov, Surface Innovations 1, 190 (2013). https://doi.org/10.1680/si.12.00018.
S. Balakrishnan and R. Nagarajan, International Journal of Advances in Engineering Sciences and Applied Mathematics 6, 41 (2014). https://doi.org/10.1007/s12572-014-0108-8.
Y. Laxminarayan, P. A. Jensen, H. Wu, F. J. Frandsen, B. Sander, and P. Glarborg, Energy & Fuels 31, 8733 (2017). https://doi.org/10.1021/acs.energyfuels.7b01312.
Y. Laxminarayan, et al., Energy & Fuels 32, 4432 (2018). https://doi.org/10.1021/acs.energyfuels.7b03114.
H. Naganuma, et al., Proceedings of the Combustion Institute 32, 2709 (2009). https://doi.org/10.1016/j.proci.2008.06.001.
C. Cunha, N. Lima, J. Martinelli, A. Bressiani, A. Padial, and L. Ramanathan, Materials Research 11, 137 (2008). https://doi.org/10.1590/S1516-14392008000200005.
S. Goel, S. Björklund, N. Curry, U. Wiklund, and S. V. Joshi, Surface and Coatings Technology 315, 80 (2017). https://doi.org/10.1016/j.surfcoat.2017.02.025.
M. Shi, Z. Xue, Z. Zhang, X. Ji, E. Byon, and S. Zhang, Surface and Coatings Technology 395, 125913 (2020). https://doi.org/10.1016/j.surfcoat.2020.125913.
W. Uczak de Goes, N. Markocsan, M. Gupta, R. Vaßen, T. Matsushita, and K. Illkova, Surface and Coatings Technology 396, 125950 (2020). https://doi.org/10.1016/j.surfcoat.2020.125950.
P. Fauchais, Journal of Physics D: Applied Physics 37, R86 (2004). https://doi.org/10.1088/0022-3727/37/9/r02.
H. Salimi Jazi, eds. Advanced plasma spray applications (2012).
P. Sokołowski, L. Pawłowski, D. Dietrich, T. Lampke, and D. Jech, Journal of Thermal Spray Technology 25, 94 (2016). https://doi.org/10.1007/s11666-015-0310-7.
K. VanEvery, et al., Journal of Thermal Spray Technology 20, 817 (2011). https://doi.org/10.1007/s11666-011-9632-2.
N. Sharifi, M. Pugh, C. Moreau, and A. Dolatabadi, Surface and Coatings Technology 289, 29 (2016). https://doi.org/10.1016/j.surfcoat.2016.01.029.
B. Bhushan and Y. C. Jung, Progress in Materials Science 56, 1 (2011). https://doi.org/10.1016/j.pmatsci.2010.04.003.
Y. Guo, et al., Advanced Science 10, 2205156 (2023). https://doi.org/10.1002/advs.202205156.
P. Fauchais, M. Vardelle, A. Vardelle, and S. Goutier, Journal of Thermal Spray Technology 24, 1120 (2015). https://doi.org/10.1007/s11666-015-0286-3.
O. Aranke, W. Algenaid, S. Awe, and S. Joshi, Coatings 9, 552 (2019).
Acknowledgements
The authors acknowledge the support from Australia Research Council (ARC). The ARC training centre in Surface Engineering for Advanced Materials, SEAM, has been funded under the ARC Industrial Transformation Training Centre (ITTC) scheme via Award IC 180100005. We are grateful for the additional support of the industrial, university and other organisation partners who have contributed to the establishment and support of SEAM.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
K.K. wrote the manuscript and prepared figures S.S. reviewed the manuscript J.J. reviewed the manuscript C.C.B. reviewed the manuscript A.A. reviewed the manuscript
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organisations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khantisopon, K., Singh, S., Jitputti, J. et al. High Temperature Corrosion Resistant and Anti-slagging Coatings for Boilers: A Review. High Temperature Corrosion of mater. (2024). https://doi.org/10.1007/s11085-024-10251-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11085-024-10251-0