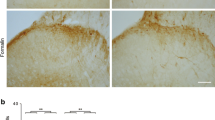
We examined the effects of propentofylline (PPF) injected intracerebroventricularly (i.c.v., 30 mM, 10 μl) into female Sprague–Dawley rats on pain responses in the formalin test and on the number of glial fibrillary acidic protein-immunopositive (GFAP-ip) astrocytes in the caudatoputamen (CPu) and periaqueductal grey (PAG) of these animals. The mean durations of flinch and lifting/biting of the limb in the PPF group vs. the vehicle (normal saline) group within phase 1 of the pain response were 280.0 ± ± 71.6 vs. 401.0 ± 69.0 sec and 69.5 ± 34.8 vs. 145.5 ± 18.6 sec, respectively (P > 0.05 in both cases, n = 7). During phase 2, the respective figures were 152.6 ± 104.0 vs. 1602.7 ± 100.9 sec and 79.1 ± ± 69.1 vs. 376.1 ± 56.5 sec (P < 0.01 in both cases). The mean numbers of GFAP-positive astrocytes per slice observed in the PPF and vehicle groups in the CPu were 35 ± 3.1 vs. 55 ± 1.9 (P < 0.01, n = 7), and those in the PAG were 30 ± 2.2 vs. 49 ± 1.2 (P < 0.01, n = 11). Thus, i. c.v. administration of PPF suppresses inflammatory pain induced by formalin injection in rats; there are reasons to believe that glial cells (astrocytes) in certain brain structures are intensely involved in the formation of a sensation of inflammatory pain.
Similar content being viewed by others
References
P. Dublin and M. Hanani, “Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain,” Brain. Behav. Immun., 21, No. 5, 592–598 (2007).
S. Maeda, A. Kawamoto, Y. Yatani, et al., “Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats,” Mol. Pain., 4, 65 (2008).
L. Vitkovic, J. Bockaert, and C. Jacque, “Inflammatory” cytokines: neuromodulators in normal brain? J. Neurochem., 74, No. 2, 457–471 (2000).
Y. S. Deng, J. H. Zhong, and X. F. Zhou, “Effects of endogenous neurotrophins on sympathetic sprouting in the dorsal root ganglia and allodynia following spinal nerve injury,” Exp. Neurol., 164, No. 2, 344–350 (2000).
X. F. Zhou, Y. S. Deng, C. J. **an, et al., “Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats,” Eur. J. Neurosci., 12, No. 1, 100–105 (2000).
H. U. Saragovi and K. Gehring, “Development of pharmacological agents for targeting neurotrophins and their receptors,” Trends. Pharmacol. Sci., 21, No. 3, 93–98 (2000).
F. E. Parkinson, A. R. Paterson, J. D. Young, et al., “Inhibitory effects of propentofylline on [3H]adenosine influx. A study of three nucleoside transport systems,” Biochem. Pharmacol., 46, No. 5, 891–896 (1993).
T. Ohkubo, Y. Mitsumoto, and T. Mohri, “Characterization of the uptake of adenosine by cultured rat hippocampal cells and inhibition of the uptake by xanthine derivatives,” Neurosci. Lett., 133, No. 2, 275–278 (1991).
M. Yao, X. Y. Chang, Y. X. Chu, et al., “Antiallodynic effects of propentofylline Elicited by interrupting spinal glial function in a rat model of bone cancer pain,” J. Neurosci. Res., 89, No. 11, 1877–1886 (2011).
V. L. Tawfik, M. R. Regan, C. Haenggeli, et al., “Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection,” Neuroscience, 152, No. 4, 1086–1092 (2008).
G. E. Ringheim, “Glial modulating and neurotrophic properties of propentofylline and its application to Alzheimer’s disease and vascular dementia,” Ann. N. Y. Acad. Sci., 903, 529–534 (2000).
G. Paxinos, C. Watson, M. Pennisi, et al., “Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight,” J. Neurosci. Methods, 13, No. 2, 139–143 (1985).
Y. K. Han, S. H. Lee, H. J. Jeong, et al., “Analgesic effects of intrathecal curcumin in the rat formalin test,” Korean. J. Pain, 25, No. 1, 1–6 (2012).
J. E. Torres-Lopez, M. I. Ortiz, G. Castaneda-Hernandez, et al., “Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test,” Life Sci., 70, No. 14, 1669–1676 (2002).
D. A. Cockayne, S. G. Hamilton, Q. M. Zhu, et al., “Urinary bladder hyporeflexia and reduced pain-related behavior in P2X3-deficient mice,” Nature, 407, No. 6807, 1011–1015 (2000).
A. M. Aloisi and I. Ceccarelli, “Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration,” Neuroscience, 95, No. 2, 559–566 (2000).
S. C. Kao, X. Zhao, C. Y. Lee, et al., “Absence of μ opioid receptor mRNA expression in astrocytes and microglia of rat spinal cord, ” NeuroReport, 18, 378–384 (2012).
J. Bachynsky, P. McCracken, D. Lier, et al., “Propentofylline treatment for Alzheimer disease and vascular dementia: an economic evaluation based on functional abilities,” Alzheimer Dis. Assoc. Disord, 14, 102–111 (2000).
B. Kittner, “Clinical trials of propentofylline in vascular dementia. European/Canadian propentofylline study group,” Alzheimer Dis. Assoc. Disord, 13, No. 3, 166–171 (1999).
S. M. Sweitzer, P. Schubert, J. A. DeLeo, “Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain,” J. Pharmacol. Exp. Ther., 297, No. 3, 1210–1217 (2001).
M. Dorazil-Dudzik, J. Mika, M. K. Schafer, et al., “The effects of local pentoxifylline and propentofylline treatment on formalin-induced pain and tumor necrosis factor-alpha messenger RNA levels in the inflamed tissue of the rat paw,” Anesth. Analg., 98, No. 6, 1566–1573 (2004).
S. D. Shields, D. J. Cavanaugh, H. Lee, et al., “Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors,” Pain, 151, No. 2, 422–429 (2010).
E. Eisenberg, B. P. Vos, and A. M. Strassman, “The NMDA antagonist Memantine blocks pain behavior in a rat model of formalin-induced facial pain,” Pain, 54, No. 3, 301–307 (1993).
K. Wiech, M. Ploner, and I. Tracey, “Neurocognitive aspects of pain perception,” Trends Cogn. Sci., 12, No. 8, 306–313 (2008).
J. Kong, P. C. Tu, C. Zyloney, et al., “Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study,” Behav. Brain Res., 211, No. 2, 215–219 (2010).
A. P. Wunderlich, R. Klug, G. Stuber, et al., “Caudate nucleus and insular activation during a pain suppression paradigm comparing thermal and electrical stimulation,” Open Neuroimag J., 5, 1–8 (2011).
D. R. Loyd and A. Z. Murphy, “The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different?” Neural. Plast., 2009, 462879 (2009).
B. Ying, N. Lu, Y. Q. Zhang, et al., “Involvement of spinal glia in tetanically sciatic stimulation-induced bilateral mechanical allodynia in rats,” Biochem. Biophys. Res. Commun, 340, 1264–1272 (2006).
L. L. Liang, J. L. Yang, N. Lu, et al., “Synergetic analgesia of propentofylline and electroacupuncture by interrupting spinal glial function in rats,” Neurochem. Res., 35, No. 11, 1780–1786 (2010).
S. M. Sweitzer, P. Schubert, and J. A. DeLeo, “Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain,” J. Pharmacol. Exp. Ther., 297, 1210–1217 (2001).
H. Cao and Y. Q. Zhang, “Spinal glial activation contributes to pathological pain states,” Neurosci. Biobehav. Rev., 32, 972–983 (2008).
L. R. Watkins, E. D. Milligan, and S. F. Maier, “Glial activation: a driving force for pathological pain,” Trends Neurosci., 24, 450–455 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, ML., Yu, HX., Tian, J. et al. Attenuation of Formalin-Induced Inflammatory Nociception by Propentofylline: Modulation of Glia. Neurophysiology 44, 441–447 (2012). https://doi.org/10.1007/s11062-012-9315-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-012-9315-8