Abstract
Background
First-line therapies for medulloblastoma(MBL) are obtaining higher survival-rates while decreasing late-effects, but treatment at relapse is not standardized. We report here the experience with MBL re-irradiation(re-RT), its timing and outcome in different clinical settings and tumor groups.
Methods
Patient’s staging/treatment at diagnosis, histotypes/molecular subgroups, relapse site/s, re-treatments outcome are reported.
Results
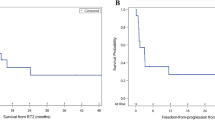
25 patients were included, with a median age of 11.4 years; 8 had metastases. According to 2016–2021 WHO-classification, 14 had SHH subgroup tumors(six TP53 mutated,one + MYC,one + NMYC amplification), 11 non-WNT/non-SHH (two with MYC/MYCN amplification).Thirteen had received HART-CSI, 11 standard-CSI, one HFRT; all post-radiation chemotherapy(CT), 16 also pre-RT. Median time to relapse (local-LR in nine, distant-DR in 14, LR + DR in two) was 26 months. Fourteen patients were re-operated, in five cases excising single DR-sites, thereafter three received CT, two after re-RT; out of 11 patients not re-operated, four had re-RT as first treatment and seven after CT. Re-RT was administered at median 32 months after first RT: focally in 20 cases, craniospinal-CSI in five. Median post-relapse-PFS/after re-RT was 16.7/8.2 months, while overall survival-OS was 35.1/23.9 months, respectively. Metastatic status both at diagnosis/relapse negatively affected outcome and re-surgery was prognostically favorable. PD after re-RT was however significantly more frequent in SHH (with a suggestive association with TP53 mutation, p = 0.050). We did not observe any influence of biological subgroups on PFS from recurrence while SHH showed apparently worse OS compared to non-WNT/non-SHH group.
Conclusions
Re-surgery + reRT can prolong survival; a substantial fraction of patients with worse outcome belongs to the SHH-subgroup.
Highlights
Medulloblastoma relapse occurs in 30% of patients and no standard therapeutic strategy exists. Surgical treatment of relapse may improve prognosis.
Reirradiation can prolong survival, however field extension, doses and fractionation have not yet been determined.
Relapsing patients with SHH subgroup medulloblastoma have a worse prognosis and may be preferentially treated with hypofractionated schedules.


Similar content being viewed by others
Data Availability
Data are available upon request sent to the first author.
References
Gajjar A, Chintagumpala M, Ashley D et al (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7:813–820 https://doi.org/10.1016/S1470-2045(06)70867-1
Massimino M, Biassoni V, Gandola L et al (2016) Childhood medulloblastoma. Crit Rev Oncol Hematol 105:35–51. https://doi.org/10.1016/j.critrevonc.2016.05.012
Dufour C, Foulon S, Geoffray A et al (2021) Prognostic relevance of clinical and molecular risk factors in children with high-risk medulloblastoma treated in the phase II trial PNET HR + 5. Neuro Oncol 23:1163–1172. https://doi.org/10.1093/neuonc/noaa301
Gandola L, Massimino M, Cefalo G et al (2009) Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. J Clin Oncol 27:566–571. https://doi.org/10.1200/JCO.2008.18.4176
Massimino M, Gandola L, Spreafico et al (2009) No salvage using high-dose chemotherapy plus/minus reirradiation for relapsing previously irradiated medulloblastoma. Int J Radiat Oncol Biol Phys 73:1358–1363. https://doi.org/10.1016/j.ijrobp.2008.06.1930
Sabel M, Fleischhack G, Tippelt S, SIOP-E Brain Tumour Group et al (2016) Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol 129:515–524. https://doi.org/10.1007/s11060-016-2202-1
Kieran MW, Chisholm J, Casanova M et al (2017) Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro Oncol 19:1542–1552. https://doi.org/10.1093/neuonc/nox109
Slavc I, Mayr L, Stepien N, Gojo J et al (2022) Improved long-term survival of patients with recurrent Medulloblastoma treated with a “MEMMAT-like. Metronomic Antiangiogenic Approach Cancers (Basel) 14:5128. https://doi.org/10.3390/cancers14205128
Donovan LK, Delaidelli A, Joseph SK et al (2020) Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med 26:720–731. https://doi.org/10.1038/s41591-020-0827-2
Winnicki C, Leblond P, Bourdeaut F, Pagnier A et al (2023 Feb) Retrospective National “Real Life” experience of the SFCE with the metronomic MEMMAT and MEMMAT-like protocol. J Clin Med 10(4):1415. https://doi.org/10.3390/jcm12041415
Hill RM, Plasschaert SLA, Timmermann B et al (2021) Relapsed Medulloblastoma in Pre-Irradiated Patients: current practice for Diagnostics and Treatment. Cancers (Basel) 14:126. https://doi.org/10.3390/cancers14010126
Gaab C, Adolph JE, Tippelt S, Mikasch R et al (2022) Local and systemic therapy of recurrent Medulloblastomas in Children and Adolescents: results of the P-HIT-REZ 2005 study. Cancers (Basel). 14:471. https://doi.org/10.3390/cancers14030471
Baroni LV, Freytes C, Fernández Ponce N et al (2021) Craniospinal irradiation as part of re-irradiation for children with recurrent medulloblastoma. J Neurooncol 155:53–61
Tsang DS, Sarhan N, Ramaswamy V et al (2019) Re-irradiation for children with recurrent medulloblastoma in Toronto, Canada: a 20-year experience. J Neurooncol 145:107–114. https://doi.org/10.1007/s11060-019-03272-2
Gupta T, Maitre M, Sastri GJ et al (2019) Outcomes of salvage re-irradiation in recurrent medulloblastoma correlate with age at initial diagnosis, primary risk-stratification, and molecular subgrou**. J Neurooncol 144:283–291. https://doi.org/10.1007/s11060-019-03225-9
Rao AD, Rashid AS, Chen Q et al (2017) Reirradiation for Recurrent Pediatric Central Nervous System Malignancies: a multi-institutional review. Int J Radiat Oncol Biol Phys 99:634–641. https://doi.org/10.1016/j.ijrobp.2017.07.026
Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE (2014) Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer 120:3731–3737. https://doi.org/10.1002/cncr.28907
Louis DN, Perry A, Wesseling P, Brat DJ et al (2021) The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Lannering B, Rutkowski S, Doz F et al (2012) Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 30:3187–3193. https://doi.org/10.1200/JCO.2011.39.8719
Rutkowski S, von Hoff K, Emser A et al (2010) Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 28:4961–4968. https://doi.org/10.1200/JCO.2010.30.2299
Ellison DW, Dalton J, Kocak M et al (2011) Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121:381–396. https://doi.org/10.1007/s00401-011-0800-8
Aldosari N, Bigner SH, Burger PC et al (2002) MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch Pathol Lab Med 126:540–544. https://doi.org/10.5858/2002-126-0540-MAMOAI
Schwalbe EC, Williamson D, Lindsey JC et al (2013) DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol 125:359–371. https://doi.org/10.1007/s00401-012-1077-2
Avula S, Peet A, Morana G, Morgan P, Warmuth-Metz M, Jaspan T (2021) European Society for Paediatric Oncology (SIOPE)-Brain Tumour Imaging Group. European Society for Paediatric Oncology (SIOPE) MRI guidelines for imaging patients with central nervous system tumours. Childs Nerv Syst 37:2497–2508. https://doi.org/10.1007/s00381-021-05199-4
Warren KE, Vezina G, Poussaint TY et al (2018) Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology committee. Neuro Oncol 20:13–23. https://doi.org/10.1093/neuonc/nox087
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8:551–561
Massimino M, Cefalo G, Riva D et al (2012) Long-term results of combined preradiation chemotherapy and age-tailored radiotherapy doses for childhood medulloblastoma. J Neurooncol 108:163–171. https://doi.org/10.1007/s11060-012-0822-7
Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70:1350–1360. https://doi.org/10.1016/j.ijrobp.2007.08.015
Tsang DS, Laperriere NJ (2019) Re-irradiation for paediatric tumours. Clin Oncol (R Coll Radiol). 31:191–198. https://doi.org/10.1016/j.clon.2018.10.003
Bakst RL, Dunkel IJ, Gilheeney S et al (2011) Reirradiation for recurrent medulloblastoma. Cancer 117:4977–4982. https://doi.org/10.1002/cncr.26148
Massimino M, Casanova M, Polastri D, Biassoni V, Modena P, Pecori E, Schiavello E, De Pava MV, Indini A, Rampini P, Bauer D, Catania S, Podda M, Gandola L (2013 Jul) Relapse in medulloblastoma: what can be done after abandoning high-dose chemotherapy? A mono-institutional experience. Childs Nerv Syst 29(7):1107–1112. https://doi.org/10.1007/s00381-013-2104-x
Torres CF, Rebsamen S, Silber JH et al (1994) Surveillance scanning of children with medulloblastoma. N Engl J Med 330:892–895. https://doi.org/10.1056/NEJM199403313301303
Yalçin B, Büyükpamukçu M, Akalan N, Cila A, Kutluk MT, Akyüz C (2002) Value of surveillance imaging in the management of medulloblastoma. Med Pediatr Oncol 38:91–97. https://doi.org/10.1002/mpo.1278
Saunders DE, Hayward RD, Phipps KP, Chong WK, Wade AM (2003) Surveillance neuroimaging of intracranial medulloblastoma in children: how effective, how often, and for how long? J Neurosurg 99:280–286. https://doi.org/10.3171/jns.2003.99.2.0280
Minn AY, Pollock BH, Garzarella L et al (2001) Surveillance neuroimaging to detect relapse in childhood brain tumors: a Pediatric Oncology Group study. J Clin Oncol 19:4135–4140. https://doi.org/10.1200/JCO.2001.19.21.4135
Kumar R, Smith KS, Deng M et al (2021) Clinical outcomes and patient-matched molecular composition of relapsed Medulloblastoma. J Clin Oncol 39:807–821. https://doi.org/10.1200/JCO.20.01359
Garcia-Lopez J, Kumar R, Smith KS, Northcott PA (2021) Deconstructing Sonic hedgehog medulloblastoma: Molecular Subtypes, Drivers, and Beyond. Trends Genet 37:235–250. https://doi.org/10.1016/j.tig.2020.11.001
Funding
This work was supported by spontaneous donations from many Charities (Associazione Bianca Garavaglia ODV, Busto Arsizio; Con Lorenzo per Mano, Como; Bimbo tu, Bologna, Associazione Italiana per la Lotta al Neuroblastoma, Genova).
Author information
Authors and Affiliations
Contributions
Conceptualization: MM, SV, FB, FRB; Methodology: FB, MM, SV; Formal analysis: MM, SB, FB, FRB, MA, FC, SM, EP, ES, VB, ON, LB, FG, EM, PM, LDC, BP; Investigation: MM, SV, FB, FRB, LB, MA, SM; Resources: MM, SV, FB, FRB, LB, MA, SM; Data curation: MM, SV, FB, FRB, FC; Writing manuscript: All authors; Supervision: MM, SB, FB; Project administration: MM, SB, FB, FRB, LB; Funding acquisition: MM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maura Massimino and Sabina Vennarini equally contributed to this paper and are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.

11060_2023_4361_MOESM1_ESM.png
Supplementary Material 1: Supplementary Fig. 1. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) from first relapse and from the beginning of the radiotherapy at first relapse.

11060_2023_4361_MOESM2_ESM.png
Supplementary Material 2: Supplementary Fig. 2. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) from first relapse and from the beginning of the radiotherapy at first relapse according to disease localization at primary diagnosis.

11060_2023_4361_MOESM3_ESM.png
Supplementary Material 3: Supplementary Fig. 3. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) from first relapse and from the beginning of the radiotherapy at first relapse according to the presence of symptoms at first relapse.

11060_2023_4361_MOESM4_ESM.png
Supplementary Material 4: Supplementary Fig. 4. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) from first relapse and from the beginning of radiotherapy at first relapse according to disease localization at first relapse.

11060_2023_4361_MOESM5_ESM.png
Supplementary Material 5: Supplementary Fig. 5. Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS) from first relapse and from the beginning of the radiotherapy at first relapse according to patients’ MBL 2016–2021 WHO-classification.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Massimino, M., Vennarini, S., Buttarelli, F.R. et al. Optimizing reirradiation for relapsed medulloblastoma: identifying the ideal patient and tumor profiles. J Neurooncol 163, 577–586 (2023). https://doi.org/10.1007/s11060-023-04361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04361-z