Abstract
Purpose
This study aimed to explore the genetic alterations and to identify good responders in the experimental arm in the tumor samples from newly diagnosed glioblastoma (GBM) patients enrolled in JCOG0911; a randomized phase II trial was conducted to compare the efficacy of interferonβ (IFNβ) plus temozolomide (TMZ) with that of TMZ alone.
Experimental
design
Of 122 tumors, we performed deep targeted sequencing to determine the somatic mutations, copy number variations, and tumor mutation burden; pyrosequencing for O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation; Sanger sequencing for the telomerase reverse transcriptase (TERT) promoter; and microsatellite instability (MSI) testing in 95, 91, 91 and 72 tumors, respectively. We performed a multivariable Cox regression analysis using backward stepwise selection of variables including clinical factors (sex, age, performance status, residual tumor after resection, tumor location) and genetic alterations.
Results
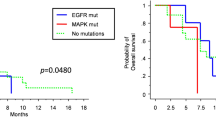
Deep sequencing detected an IDH1 mutation in 13 tumors (14%). The MGMT promoter methylation by quantitative pyrosequencing was observed in 41% of the tumors. A mutation in the TERT promoter was observed in 69% of the tumors. While high tumor mutation burden (> 10 mutations per megabase) was seen in four tumors, none of the tumors displayed MSI-high. The clinical and genetic factors considered as independent favorable prognostic factors were gross total resection (hazard ratio [HR]: 0.49, 95% confidence interval, 0.30–0.81, P = 0.0049) and MGMT promoter methylation (HR: 0.43, 0.21–0.88, P = 0.023). However, tumor location at the temporal lobe (HR: 1.90, 1.22–2.95, P = 0.0046) was an independent unfavorable prognostic factor. No predictive factors specific to the TMZ + IFNβ + Radiotherapy (RT) group were found.
Conclusion
This additional sub-analytical study of JCOG0911 among patients with newly diagnosed GBM showed that tumor location at the temporal lobe, gross total resection, and MGMT promoter methylation were significant prognostic factors, although no factors specific to IFNβ addition were identified.




Similar content being viewed by others
References
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(2):1–56. https://doi.org/10.1093/neuonc/not151
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, Organisation E, for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G, (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, E, Treatment of Cancer Brain T, Radiation Oncology G, National Cancer Institute of Canada Clinical Trials G (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Weller M, Tabatabai G, Kastner B, Felsberg J, Steinbach JP, Wick A, Schnell O, Hau P, Herrlinger U, Sabel MC, Wirsching HG, Ketter R, Bahr O, Platten M, Tonn JC, Schlegel U, Marosi C, Goldbrunner R, Stupp R, Homicsko K, Pichler J, Nikkhah G, Meixensberger J, Vajkoczy P, Kollias S, Husing J, Reifenberger G, Wick W, Group DS (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the director trial. Clin Cancer Res 21:2057–2064. https://doi.org/10.1158/1078-0432.CCR-14-2737
Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N, Stupp R (2004) Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 10:1871–1874. https://doi.org/10.1158/1078-0432.ccr-03-0384
Galani V, Papadatos SS, Alexiou G, Galani A, Kyritsis AP (2017) In Vitro and In Vivo Preclinical Effects of Type I IFNs on Gliomas. J Interferon Cytokine Res 37:139–146. https://doi.org/10.1089/jir.2016.0094
GuhaSarkar D, Su Q, Gao G, Sena-Esteves M (2016) Systemic AAV9-IFNbeta gene delivery treats highly invasive glioblastoma. Neuro Oncol 18:1508–1518. https://doi.org/10.1093/neuonc/now097
Kawaji H, Tokuyama T, Yamasaki T, Amano S, Sakai N, Namba H (2015) Interferon-beta and temozolomide combination therapy for temozolomide monotherapy-refractory malignant gliomas. Mol Clin Oncol 3:909–913. https://doi.org/10.3892/mco.2015.542
Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J (2005) IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res 65:7573–7579. https://doi.org/10.1158/0008-5472.CAN-05-0036
Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, Ito M, Yoshida J (2007) A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol 61:653–659. https://doi.org/10.1007/s00280-007-0520-x
Wakabayashi T, Natsume A, Mizusawa J, Katayama H, Fukuda H, Sumi M, Nishikawa R, Narita Y, Muragaki Y, Maruyama T, Itosan T, Beppu T, Nakamura H, Kayama T, Sato S, Nagane M, Mishima K, Nakasu Y, Kurisu K, Yamasaki F, Sugiyama K, Onishi T, Iwadate Y, Terasaki M, Kobayashi H, Matsumura A, Ishikawa E, Sasaki H, Mukasa A, Matsuo T, Hirano H, Kumabe T, Shinoura N, Hashimoto N, Aoki T, Asai A, Abe T, Yoshino A, Arakawa Y, Asano K, Yoshimoto K, Shibui S, Members of Japan Clinical Oncology Group Brain Tumor Study G (2018) JCOG0911 INTEGRA study: a randomized screening phase II trial of interferonbeta plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma. J Neurooncol 138:627–636. https://doi.org/10.1007/s11060-018-2831-7
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576. https://doi.org/10.1101/gr.129684.111
Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, Wooster R (2004) The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer 91:355–358. https://doi.org/10.1038/sj.bjc.6601894
Davis JL, Grenert JP, Horvai AE (2014) Loss of heterozygosity and ` are rare in sporadic dedifferentiated liposarcoma: a study of 43 well-characterized cases. Arch Pathol Lab Med 138:823–827. https://doi.org/10.5858/arpa.2013-0236-OA
An B, Kondo Y, Okamoto Y, Shinjo K, Kanemitsu Y, Komori K, Hirai T, Sawaki A, Tajika M, Nakamura T, Yamao K, Yatabe Y, Fujii M, Murakami H, Osada H, Tani T, Matsuo K, Shen L, Issa JP, Sekido Y (2010) Characteristic methylation profile in CpG island methylator phenotype-negative distal colorectal cancers. Int J Cancer 127:2095–2105. https://doi.org/10.1002/ijc.25225
Shinjo K, Okamoto Y, An B, Yokoyama T, Takeuchi I, Fujii M, Osada H, Usami N, Hasegawa Y, Ito H, Hida T, Fujimoto N, Kishimoto T, Sekido Y, Kondo Y (2012) Integrated analysis of genetic and epigenetic alterations reveals CpG island methylator phenotype associated with distinct clinical characters of lung adenocarcinoma. Carcinogenesis 33:1277–1285. https://doi.org/10.1093/carcin/bgs154
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57:289–300
Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, Motomura K, Ohka F, Shiina S, Yamamoto T, Nagata Y, Yoshizato T, Mizoguchi M, Abe T, Momii Y, Muragaki Y, Watanabe R, Ito I, Sanada M, Yajima H, Morita N, Takeuchi I, Miyano S, Wakabayashi T, Ogawa S, Natsume A (2018) Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol 20:66–77. https://doi.org/10.1093/neuonc/nox132
Rubin DB (2004) Multiple imputation for nonresponse in surveys. Wiley-Interscience, Hoboken, N.J.
Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, Ito M, Yoshida J (2008) A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol 61:653–659. https://doi.org/10.1007/s00280-007-0520-x
Lim SM, Kim HR, Lee JS, Lee KH, Lee YG, Min YJ, Cho EK, Lee SS, Kim BS, Choi MY, Shim HS, Chung JH, La Choi Y, Lee MJ, Kim M, Kim JH, Ali SM, Ahn MJ, Cho BC (2017) Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 35:2613–2618. https://doi.org/10.1200/JCO.2016.71.3701
Wu K, Liao X, Gong Y, He J, Zhou JK, Tan S, Pu W, Huang C, Wei YQ, Peng Y (2019) Circular RNA F-circSR derived from SLC34A2-ROS1 fusion gene promotes cell migration in non-small cell lung cancer. Mol Cancer 18:98. https://doi.org/10.1186/s12943-019-1028-9
Chen J, Wang P, Cai R, Peng H, Zhang C, Zhang M (2019) SLC34A2 promotes neuroblastoma cell stemness via enhancement of miR-25/Gsk3beta-mediated activation of Wnt/beta-catenin signaling. FEBS Open Bio 9:527–537. https://doi.org/10.1002/2211-5463.12594
Wang Y, Yang W, Pu Q, Yang Y, Ye S, Ma Q, Ren J, Cao Z, Zhong G, Zhang X, Liu L, Zhu W (2019) Correction to: The effects and mechanisms of SLC34A2 in tumorigenesis and progression of human non-small cell lung cancer. J Biomed Sci 26:18. https://doi.org/10.1186/s12929-019-0508-y
Bhagwat AS, Roe JS, Mok BYL, Hohmann AF, Shi J, Vakoc CR (2016) BET Bromodomain Inhibition Releases the Mediator Complex from Select cis-Regulatory Elements. Cell Rep 15:519–530. https://doi.org/10.1016/j.celrep.2016.03.054
Calpena E, Hervieu A, Kaserer T, Swagemakers SMA, Goos JAC, Popoola O, Ortiz-Ruiz MJ, Barbaro-Dieber T, Bownass L, Brilstra EH, Brimble E, Foulds N, Grebe TA, Harder AVE, Lees MM, Monaghan KG, Newbury-Ecob RA, Ong KR, Osio D, Reynoso Santos FJ, Ruzhnikov MRZ, Telegrafi A, van Binsbergen E, van Dooren MF, Deciphering Developmental Disorders S, van der Spek PJ, Blagg J, Twigg SRF, Mathijssen IMJ, Clarke PA, Wilkie AOM (2019) De Novo Missense Substitutions in the Gene Encoding CDK8, a Regulator of the Mediator Complex, Cause a Syndromic Developmental Disorder. Am J Hum Genet 104:709–720. https://doi.org/10.1016/j.ajhg.2019.02.006
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Lamborn KR, Chang SM, Prados MD (2004) Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol 6:227–235. https://doi.org/10.1215/S1152851703000620
Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS et al (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26:239–244. https://doi.org/10.1016/0360-3016(93)90203-8
Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y (1994) Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol 21:177–185. https://doi.org/10.1007/bf01052902
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. https://doi.org/10.1056/NEJMoa043331
Olson RA, Brastianos PK, Palma DA (2011) Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol 105:325–335. https://doi.org/10.1007/s11060-011-0594-5
Acknowledgements
The authors thank the FALCO biosystems (Kyoto, Japan) for their technical support and the members of JCOG Data Center/Operations Office (Mr. Junki Mizusawa, Dr. Keita Sasaki, and Dr. Hiroshi Katayama) for preparing this manuscript. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Chemistry for Multimolecular Crowding Biosystems” (to A.N.; JSPS KAKEN grant no. 17928985).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
TW has received research funding from Toray Co, Ltd, and MSD Co. Ltd. KS received honorarium from MSD. YN received honorarium from MSD, Chugai and Eisai, and research funding from Daiichi-Sankyo. M.N. received honorarium from MSD, Nippon-Kayaku, Chugai, Daiichi-Sankyo, AbbVie, Ono and Eisai, and research funding from Toray. RN received honorarium from MSD, Chugai, and Eisai. YA received honorarium from Nippon-Kayaku, Merck, and research funding from Merck. The other authors declare that they have no conflict of interest. All authors have registered online Self-reported COI Disclosure Statement Forms through the website for Japan Neurosurgical Society members.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Natsume, A., Aoki, K., Ohka, F. et al. Genetic analysis in patients with newly diagnosed glioblastomas treated with interferon-beta plus temozolomide in comparison with temozolomide alone. J Neurooncol 148, 17–27 (2020). https://doi.org/10.1007/s11060-020-03505-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03505-9