Abstract
Purpose
Recently, the potential value of isocitrate dehydrogenase (IDH) mutation as a prognostic marker in glioblastomas has been established. Glioblastomas are classified by their IDH mutation status under the 2016 WHO classification system. However, noninvasive diagnostic methods for the mutation status in glioblastoma patients have not been established so far. The purpose of this study was to evaluate the difference of acetate metabolism between in glioblastomas with wild-type IDH and in those with IDH mutation by comparing the uptake of 14C-acetate using genetically engineered glioblastoma cell lines in vitro and in vivo.
Methods
We established glioblastoma cells (U251) expressing IDH1 R132H and examined the cell uptake of [1-14C]acetate. Biodistribution studies and an autoradiographic study for U251 cell tumor-bearing mice (BALB/c-nu/nu) with or without the IDH1 mutation were performed 1 h after [1-14C]acetate administration.
Results
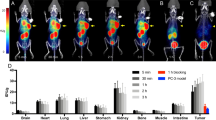
Significantly higher uptake of [1-14C]acetate was observed in U251/IDH1 R132H cells than in U251/IDH1 wild-type cells both in vitro (10.11 ± 0.94 vs. 4.26 ± 0.95%dose/mg, p = 0.0047) and in vivo (0.97 ± 0.14 vs. 0.66 ± 0.05%ID/g; p = 0.0037). Tumor-to-muscle ratios were also significantly higher in U251/IDH1 R132H tumors (3.36 ± 0.41 vs. 1.88 ± 0.59, p = 0.0030). The autoradiographic study shows the entirely higher radioactivity of the U251/IDH1 R132H tumor tissue section than that of the U251/IDH1 Wild-type tumor.
Conclusions
In vitro and in vivo studies demonstrated that the uptake of radiolabeled acetate was significantly higher in IDH-mutated cells than in IDH-wild-type cells.



Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Karlo CA, Di Paolo PL, Chaim J, Hakimi AA, Ostrovnaya I, Russo P, Hricak H, Motzer R, Hsieh JJ, Akin O (2014) Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology 270:464–471
Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y, Aldape K, Cha S, Kuo MD (2008) Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci USA 105:5213–5218
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, ** S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Yang M, Soga T, Pollard PJ (2013) Oncometabolites: linking altered metabolism with cancer. J Clin Invest 123:3652–3658
Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen, AG (2012) Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 4:116ra4
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, Pascual JM, Madden CJ, Mickey BE, Malloy CR, Bachoo RM, Maher EA (2012) 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 18:624–629
Leather T, Jenkinson MD, Das K, Poptani H (2017) Magnetic resonance spectroscopy for detection of 2-hydroxyglutarate as a biomarker for IDH mutation in gliomas. Metabolites 7:29
Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP (1978) Metabolic trap** as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med 19:1154–1161
Ponde DE, Dence CS, Oyama N, Kim J, Tai YC, Laforest R, Siegel BA, Welch MJ (2007) 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging–in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med 48:420–428
Kim S, Kim D, Kim SH, Park MA, Chang JH, Yun M (2018) The roles of 11C-acetate PET/CT in predicting tumor differentiation and survival in patients with cerebral glioma. Eur J Nucl Med Mol Imaging 45:1012–1020
Tsuchida T, Takeuchi H, Okazawa H, Tsujikawa T, Fujibayashi Y (2008) Grading of brain glioma with 1–11C-acetate PET: comparison with 18F-FDG PET. Nucl Med Biol 35:171–176
Yamamoto Y, Nishiyama Y, Kimura N, Kameyama R, Kawai N, Hatakeyama T, Kaji M, Ohkawa M (2008) 11C-acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol Imaging Biol 10:281–287
Kim D, Chun JH, Kim SH, Moon JH, Kang SG, Chang JH, Yun M (2019) Re-evaluation of the diagnostic performance of 11C-methionine PET/CT according to the 2016 WHO classification of cerebral gliomas. Eur J Nucl Med Mol Imaging 46:1678–1684
Qu W, Oya S, Lieberman BP, Ploessl K, Wang L, Wise DR, Divgi CR, Chodosh LA, Thompson CB, Kung HF (2012) Preparation and characterization of L-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med 53:98–105
Salamanca-Cardona L, Shah H, Poot AJ, Correa FM, Di Gialleonardo V, Lui H, Miloushev VZ, Granlund KL, Tee SS, Cross JR, Thompson CB, Keshari KR (2017) In vivo imaging of glutamine metabolism to the oncometabolite 2-hydroxyglutarate in IDH1/2 mutant tumors. Cell Metab 26:830–841
Cohen AL, Holmen SL, Colman H (2013) IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep 13:345
Yoshii Y, Furukawa T, Oyama N, Hasegawa Y, Kiyono Y, Nishii R, Waki A, Tsuji AB, Sogawa C, Wakizaka H, Fukumura T, Yoshii H, Fujibayashi Y, Lewis JS, Saga T (2013) Fatty acid synthase is a key target in multiple essential tumor functions of prostate cancer: uptake of radiolabeled acetate as a predictor of the targeted therapy outcome. PLoS ONE 8:e64570
Piaskowski S, Bienkowski M, Stoczynska-Fidelus E, Stawski R, Sieruta M, Szybka M, Papierz W, Wolanczyk M, Jaskolski DJ, Liberski PP, Rieske P (2011) Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer 104:968–970
Acknowledgements
We thank Ms. Christalle Chow (Graduate School of Biostudies, Kyoto University) for manuscript editing and her useful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The present study was financially supported by JSPS KAKENHI Grant Number JP 19K17164. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No other potential conflicts of interest relevant to this article exist.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. We also followed the ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines) for animal researches.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2019_3322_MOESM1_ESM.tif
Supplemental Fig. 1. (a) Western blot of cell lysates from the stably transfected HeLa clones, HeLa/IDH1 R132H, demonstrates the expression of mutated IDH1 protein fused to the V5 peptide. (b) The cellular uptake value of 14C-acetate was examined and expressed as a percentage of the added dose per total protein of the cell lysates. Supplemental Fig. 2. The volume and weight of xenografts were measured 14 days after transplantation with the indicated two stable transfectants, U251/IDH1 Wild-type and U251/IDH1 R132H. Neither volume (a) nor weight (b) was significantly different between the two tumor xenografts, which indicated that the introduction of mutated IDH (R132H) did not affect tumor growth. (TIF 1114 kb)
Rights and permissions
About this article
Cite this article
Koyasu, S., Shimizu, Y., Morinibu, A. et al. Increased 14C-acetate accumulation in IDH-mutated human glioblastoma: implications for detecting IDH-mutated glioblastoma with 11C-acetate PET imaging. J Neurooncol 145, 441–447 (2019). https://doi.org/10.1007/s11060-019-03322-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03322-9