Abstract
Background
Helicobacter pylori is a fastidious pathogen that is required a complicated medium for growth. Invading epithelial cells of the stomach. H. pylori virulence factors are classified by function, acidic resistivity, adhesion, chemotaxis and motility, molecular mimicry, immunological invasion and modulation, and toxins formation such as cytotoxin-associated genes A (cagA) and vacuolating cytotoxin A (vacA). This study aims to determine a simple and innovative technique to isolate H. pylori from gastric biopsies and assess pathogenicity by virulence factor gene detection.
Methods
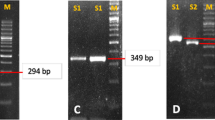
A total of 200 patients who were suspected of having H. pylori infection had two antral gastric biopsies undertaken. A rapid urease test (RUT) was used for one, and Brain Heart Infusion broth (BHI) was used to cultivate the other. The molecular study included diagnostics utilizing the 16sRNA housekee** gene along with the identification of the virulence factors genes (cagA, cagT, and vacA) and sequencing,
Result
Of the 200 antral gastric biopsies collected, 135 were positive rapid urease tests, and 17 H. pylori isolates were successfully obtained from 135 biopsies. The 16SrRNA as a housekee** gene is confirmed, and about 53%, 70.5%, and 82.3% of the 17 isolates show carrying cagA, cagT, and vacA genes, respectively. All peptic ulcer isolates have the cagA gene, while Gastroesophageal Reflux Disease (GERD) and non-peptic ulcer disease (NPUD) isolates show the lack of the cagA gene. All bacteria, which were isolated from peptic ulcer, nodular gastritis, and gastritis patients, have a vacA gene.
Conclusion
The effective method for isolating H. pylori is centrifuging the transport broth after 24 h of incubation. The cagA toxin causes peptic ulcer while vacA toxin induces several histopathological changes in the stomach. Three virulence genes were present in all peptic ulcer-causing bacteria, while only one or none were present in the GERD and NPUD biopsy isolates.




Similar content being viewed by others
Data availability
No Data associated in the manuscript.
References
Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VW, Wu JC (2017) Global prevalence of Helicobacter pylori Infection: systematic review and meta-analysis. Gastroenterology 153:420–429
Mărginean CO, Meliț, LE, Săsăran MO (2022) Traditional and modern diagnostic approaches in diagnosing Pediatric Helicobacter pylori Infection. Children 9:994
Galoș F, Boboc C, Ieșanu M-I, Anghel M, Ioan A, Iana E, Coșoreanu MT, Boboc AA (2023) Antibiotic Resistance and therapeutic efficacy of Helicobacter pylori Infection in Pediatric Patients—A Tertiary Center Experience. Antibiotics 12:146
Doohan D, Rezkitha YAA, Waskito LA, Yamaoka Y, Miftahussurur M (2021) Helicobacter pylori BabA–SabA key roles in the adherence phase: the synergic mechanism for successful colonization and Disease development. Toxins 13:485
Ansari S, Yamaoka Y (2020) Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA)-mediated gastric pathogenicity. Int J Mol Sci 21:7430
Rezaei F, Alebouyeh M, Mirbagheri SZ, Ebrahimi A, Foroushani AR, Bakhtiari R (2023) Transcriptional analysis of Helicobacter pylori cytotoxic-associated gene-pathogenicity island in response to different pH levels and proton pump inhibitor exposure. Indian J Gastroenterol, 1–8
Lin AS, Dooyema SD, Frick-Cheng AE, Harvey ML, Suarez G, Loh JT, McDonald WH, McClain MS, Peek RM Jr, Cover TL (2020) Bacterial energetic requirements for Helicobacter pylori Cag type IV secretion system-dependent alterations in gastric epithelial cells. Infect Immun 88. https://doi.org/10.1128/iai.00790-19
Chung JM, Sheedlo MJ, Campbell AM, Sawhney N, Frick-Cheng AE, Lacy DB, Cover TL, Ohi MD (2019) Structure of the Helicobacter pylori Cag type IV secretion system. Elife 8, e47644
Zhang X, Li C, Chen D, He X, Zhao Y, Bao L, Wang Q, Zhou J, **e Y (2022) H. Pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflamm Res, 1–15
McClain MS, Beckett AC, Cover TL (2017) Helicobacter pylori vacuolating toxin and gastric cancer. Toxins 9:316
Reynolds DJ, Penn CW (1994) Characteristics of Helicobacter pylori growth in a defined medium and determination of its amino acid requirements. Microbiology 140:2649–2656
Nguyen L, Uchida T, Tsukamoto Y, Kuroda A, Okimoto T, Kodama M, Murakami K, Fujioka T, Moriyama M (2010) Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin Microbiol Infect 16:1264–1269
Brkić DV, Katičić M, Bedenić B, Stanko, AP, Plečko V (2016) Detection of virulence gene belonging to cag pathogenicity island in Helicobacter pylori isolates after multiple unsuccessful eradication therapy in Northwest Croatia. Periodicum biologorum 118
Nagata R, Sato H, Takenaka S, Yokoyama J, Terai S, Mimuro H, Noiri Y (2023) Analysis of genetic relatedness between gastric and oral Helicobacter pylori in patients with early gastric Cancer using Multilocus sequence ty**. Int J Mol Sci 24:2211
Godbole G, Mégraud F, Bessède E (2020) Diagnosis of Helicobacter pylori Infection. Helicobacter 25:e12735
Seo J-H, Park JS, Rhee KH, Youn H-S (2015) Limitations of urease test in diagnosis of pediatric Helicobacter pylori Infection. World J Clin Pediatr 4:143
Hussen BM, Qader SS, Ahmed HF, Ahmed SH (2013) The prevalence of Helicobacter pylori among university students in Iraq. Indian J Sci Technol 6:5019–5023
Kaplan M, Tanoglu A, Duzenli T, Tozun AN (2019) Helicobacter pylori treatment in Turkey: current status and rational treatment options. North Clin Istanbul 7:87–94
Hakami OA, Alsubaie RA, Albaqami BA, Almutlaq HM, Alqahtani NM, Alkhonezan M, Almuqrin FF, Alghamdi AH, Alaryni AA, Qutob RA (2023) Knowledge and perception of Physicians of different specialties in Saudi Arabia toward Helicobacter pylori. J Multidisciplinary Healthc, 763–771
Khoder G, Muhammad JS, Mahmoud I, Soliman SS, Burucoa C (2019) Prevalence of Helicobacter pylori and its associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens 8:44
Patel SK, Mishra GN, Pratap CB, Jain AK, Nath G (2014) Helicobacter pylori is not eradicated after triple therapy: a nested PCR based study. BioMed research international 2014
Malfertheiner P, Megraud F, O’morain C, Gisbert J, Kuipers E, Axon A, Bazzoli F, Gasbarrini A, Atherton J, Graham DY (2017) Management of Helicobacter pylori Infection—the Maastricht V/Florence consensus report. Gut 66:6–30
Metwally M, Sayed MM, Abbas AH, El Shahat ESA, Elkholy H (2023) Helicobacter pylori Culture and Anti-biogram: low yield and beneficial results. Benha Medical Journal
Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G (2014) Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterology: WJG 20:12847
Mégraud F, Lehours P (2007) Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 20:280–322
Khadangi F, Yassi M, Kerachian MA (2017) Diagnostic accuracy of PCR-based detection tests for Helicobacter Pylori in stool samples. Helicobacter 22, e12444
Moorlag SJ, Coolen JP, van den Bosch B, ** EH-M, Buil JB, Wertheim HF, Melchers WJ (2023) Targeting the 16S rRNA gene by reverse complement PCR next-generation sequencing: specific and sensitive detection and identification of Microbes directly in clinical samples. Microbiol Spectr 11:e04483–e04422
Elnosh M, Altayb H, Hamedelnil Y, Elshareef W, Abugrain A, Osman E, Albasha A, Abdelhamid A, Moglad E, AbdAlla A (2022) Comparison of invasive histological and molecular methods in the diagnosis of Helicobacter pylori from gastric biopsies of Sudanese patients: a cross-sectional study. F1000Research 11
AL-Ouqaili MT, Abdullah EM (2011) Detection of 16S r RNA gene of Helicobacter pylori in patients with Peptic Ulcer and gastric carcinoma: molecular and bacteriological study. Egypt Acad J Biol Sci G Microbiol 3:95–104
Feliciano O, Gutierrez O, Valdés L, Fragoso T, Calderin AM, Valdes AE, Llanes R (2015) Prevalence of Helicobacter pylori vacA, cagA, and iceA genotypes in Cuban patients with upper Gastrointestinal Diseases. Biomed Res Int 2015:1–6
Johnson EM, Gaddy JA, Voss BJ, Hennig EE, Cover TL (2014) Genes required for assembly of pili associated with the Helicobacter pylori cag type IV secretion system. Infect Immun 82:3457–3470
Sallas ML, Melchiades JL, Zabaglia LM, do Prado Moreno JR, Orcini WA, Chen ES, Smith MAC, Payão SLM, Rasmussen LT (2017) Prevalence of Helicobacter pylori vacA, cagA, dupA and oipA genotypes in patients with gastric Disease. Adv Microbiol 7:1–9
Boonyanugomol W, Kongkasame W, Palittapongarnpim P, Baik S-C, Jung M-h, Shin M-K, Kang H-L, Lee W-K (2020) Genetic variation in the cag pathogenicity island of Helicobacter pylori strains detected from gastroduodenal patients in Thailand. Brazilian J Microbiol 51:1093–1101
Hussein NR, Mohammadi M, Talebkhan Y, Doraghi M, Letley DP, Muhammad MK, Argent RH, Atherton JC (2008) Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. Pylori-associated Disease. J Clin Microbiol 46:1774–1779
Sezikli M, Guliter S, Apan T, Aksoy A, Keles H, Ozkurt Z (2006) Frequencies of serum antibodies to Helicobacter pylori CagA and VacA in a Turkish population with various gastroduodenal Diseases. Int J Clin Pract 60:1239–1243
Li Q, Liu J, Gong Y, Yuan Y (2016) Serum VacA antibody is associated with risks of Peptic Ulcer and gastric cancer: a meta-analysis. Microb Pathog 99:220–228
Censini S, Lange C, **ang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proceedings of the National Academy of Sciences 93, 14648–14653
McMahon B, Bruce M, Koch A, Goodman K, Tsukanov V, Mulvad G, Borresen M, Sacco F, Barrett D, Westby S (2016) The diagnosis and treatment of Helicobacter pylori Infection in Arctic regions with a high prevalence of Infection: Expert Commentary. Epidemiol Infect 144:225–233
Miernyk KM, Bruden D, Rudolph KM, Hurlburt DA, Sacco F, McMahon BJ, Bruce MG (2020) Presence of cagPAI genes and characterization of Vaca s, i and m regions in Helicobacter pylori isolated from alaskans and their association with clinical pathologies. J Med Microbiol 69:218–227
Monroy FP, Brown HE, Sanderson PR, Jarrin G, Mbegbu M, Kyman S, Harris RB (2022) Helicobacter pylori in native americans in northern Arizona. Diseases 10:19
Acknowledgements
The authors gratitude to the members of the RES Laboratory in the physics department and the members of the Molecular Biology Laboratory in the biology department, college of sciences, Mustansiriyah University, Baghdad, Iraq for their efficient collaboration. Utmost thanks and appreciation to the doctors and nursing staff in the Gastroenterology Division, Endoscopy Unit, Baquba Teaching Hospital for their efforts in obtaining gastric biopsies. Also, the authors would like to thank Mustansiriyah University (https://uomustansiriyah.edu.iq/) / Baghdad, Iraq for its support to complete this work.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed equally in writing—original draft preparation, all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of the gastroenterology division a Baqubah Teaching Hospital. The study was conducted and samples were collected after receiving approval from the Mustansiriyah University /College of Science’s research tasks and ethics committee on January 1, 2022 (Ref.: BCSMU/1221/0004 M). This study involved the H. pylori’s from patients who are suffering from infections at Gastroenterology Division, Endoscopy Unit, Baqubah Teaching Hospital, Iraq. All authors agree to publish this work.
Research involving human participants and/or animals
This study involved the H. pylori’s from patients who are suffering from infections at Baghdad hospitals, Baghdad, Iraq.
Informed consent
All patients gave their written informed consents before inclusion, written informed consent has been obtained from the patient(s) to publish this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Jumaily, A.Y., Al-Haddad, A. & Al-jubori, S.S. New strategies for Helicobacter pylori isolation and sequencing analysis for virulence genes contributing to its pathogenicity. Mol Biol Rep 51, 95 (2024). https://doi.org/10.1007/s11033-023-09038-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09038-4