Abstract
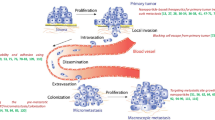
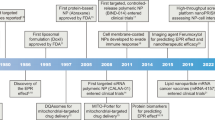
Cancer metastasis is the leading cause of cancer-related death. Metastasis occurs at all stages of tumor development, with unexplored changes occurring at the primary site and distant colonization sites. The growing understanding of the metastatic process of tumor cells has contributed to the emergence of better treatment options and strategies. This review summarizes a range of features related to tumor cell metastasis and nanobased drug delivery systems for inhibiting tumor metastasis. The mechanisms of tumor metastasis in the ideal order of metastatic progression were summarized. We focus on the prominent role of nanocarriers in the treatment of tumor metastasis, summarizing the latest applications of nanocarriers in combination with drugs to target important components and processes of tumor metastasis and providing ideas for more effective nanodrug delivery systems.








Similar content being viewed by others
Data availability
No datasets have been generated in this review, so no data availability statement is included.
Code availability
Not applicable.
References
Ganesh K, Massagué J (2021) Targeting metastatic cancer. Nat Med 27(1):34–44. https://doi.org/10.1038/s41591-020-01195-4
Hu Z, Curtis C (2020) Looking backward in time to define the chronology of Metastasis. Nat Commun 11(1):3213. https://doi.org/10.1038/s41467-020-16995-y
Graham TA, Shibata D (2020) Navigating the path to distant Metastasis. Nat Genet 52(7):642–643. https://doi.org/10.1038/s41588-020-0660-z
Suhail Y, Cain MP, Vanaja K, Kurywchak PA, Levchenko A, Kalluri R, Kshitiz (2019) Systems Biology of Cancer Metastasis. Cell Syst 9(2):109–127. https://doi.org/10.1016/j.cels.2019.07.003
Park M, Kim D, Ko S, Kim A, Mo K, Yoon H (2022) Breast cancer metastasis: mechanisms and therapeutic implications. Int J Mol Sci. https://doi.org/10.3390/ijms23126806
Gavish A, Tyler M, Greenwald AC, Hoefflin R, Simkin D, Tschernichovsky R, Galili Darnell N, Somech E, Barbolin C, Antman T, Kovarsky D, Barrett T, Gonzalez Castro LN, Halder D, Chanoch-Myers R, Laffy J, Mints M, Wider A, Tal R, Spitzer A, Hara T, Raitses-Gurevich M, Stossel C, Golan T, Tirosh A, Suvà ML, Puram SV, Tirosh I (2023) Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618(7965):598–606. https://doi.org/10.1038/s41586-023-06130-4
Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z (2018) Tumour heterogeneity and Metastasis at single-cell resolution. Nat Cell Biol 20(12):1349–1360. https://doi.org/10.1038/s41556-018-0236-7
Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15(2):81–94. https://doi.org/10.1038/nrclinonc.2017.166
Biswas D, Birkbak NJ, Rosenthal R, Hiley CT, Lim EL, Papp K, Boeing S, Krzystanek M, Djureinovic D, La Fleur L, Greco M, Döme B, Fillinger J, Brunnström H, Wu Y, Moore DA, Skrzypski M, Abbosh C, Litchfield K, Al Bakir M, Watkins TBK, Veeriah S, Wilson GA, Jamal-Hanjani M, Moldvay J, Botling J, Chinnaiyan AM, Micke P, Hackshaw A, Bartek J, Csabai I, Szallasi Z, Herrero J, McGranahan N, Swanton C (2019) A clonal expression biomarker associates with Lung cancer mortality. Nat Med 25(10):1540–1548. https://doi.org/10.1038/s41591-019-0595-z
Gupta PB, Pastushenko I, Skibinski A, Blanpain C, Kuperwasser C (2019) Phenotypic plasticity: driver of Cancer initiation, progression, and Therapy Resistance. Cell Stem Cell 24(1):65–78. https://doi.org/10.1016/j.stem.2018.11.011
Wang SE (2022) Extracellular vesicles in cancer therapy. Semin Cancer Biol 86(Pt 2):296–309. https://doi.org/10.1016/j.semcancer.2022.06.001
Shi ZD, Pang K, Wu ZX, Dong Y, Hao L, Qin JX, Wang W, Chen ZS, Han CH (2023) Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct Target Ther 8(1):113. https://doi.org/10.1038/s41392-023-01383-x
Pérez-González A, Bévant K, Blanpain C (2023) Cancer cell plasticity during Tumor progression, Metastasis and response to therapy. Nat Cancer 4(8):1063–1082. https://doi.org/10.1038/s43018-023-00595-y
Prasetyanti PR, Medema JP (2017) Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer 16(1):41. https://doi.org/10.1186/s12943-017-0600-4
Zhang M, Liu ZZ, Aoshima K, Cai WL, Sun H, Xu T, Zhang Y, An Y, Chen JF, Chan LH, Aoshima A, Lang SM, Tang Z, Che X, Li Y, Rutter SJ, Bossuyt V, Chen X, Morrow JS, Pusztai L, Rimm DL, Yin M, Yan Q (2022) CECR2 drives Breast cancer Metastasis by promoting NF-κB signaling and macrophage-mediated immune suppression. Sci Transl Med 14(630):eabf5473. https://doi.org/10.1126/scitranslmed.abf5473
Zhou HZ, Li F, Cheng ST, Xu Y, Deng HJ, Gu DY, Wang J, Chen WX, Zhou YJ, Yang ML, Ren JH, Zheng L, Huang AL, Chen J (2022) DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC Metastasis. Hepatology 75(4):847–865. https://doi.org/10.1002/hep.32195
Liang Z, Liu H, Zhang Y, **ong L, Zeng Z, He X, Wang F, Wu X, Lan P (2021) Cyr61 from adipose-derived stem cells promotes Colorectal cancer Metastasis and vasculogenic mimicry formation via integrin α(V) β(5). Mol Oncol 15(12):3447–3467. https://doi.org/10.1002/1878-0261.12998
Alotaibi AG, Li JV, Gooderham NJ (2021) Tumour necrosis factor-α (TNF-α) enhances dietary carcinogen-induced DNA damage in Colorectal cancer epithelial cells through activation of JNK signaling pathway. Toxicology 457:152806. https://doi.org/10.1016/j.tox.2021.152806
Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I (2020) The dual role of Tumor necrosis factor-alpha (TNF-α) in Breast cancer: molecular insights and therapeutic approaches. Cell Oncol 43(1):1–18. https://doi.org/10.1007/s13402-019-00489-1
Hisada Y, Mackman N (2019) Tissue factor and Cancer: regulation, Tumor Growth, and Metastasis. Semin Thromb Hemost 45(4):385–395. https://doi.org/10.1055/s-0039-1687894
Bakshi HA, Quinn GA, Nasef MM, Mishra V, Aljabali AAA, El-Tanani M, Serrano-Aroca Á, Webba Da Silva M, McCarron PA, Tambuwala MM (2022) Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-kB/VEGF pathways. Cells https://doi.org/10.3390/cells11091502
Beck TN, Boumber YA, Aggarwal C, Pei J, Thrash-Bingham C, Fittipaldi P, Vlasenkova R, Rao C, Borghaei H, Cristofanilli M, Mehra R, Serebriiskii I, Alpaugh RK (2019) Circulating Tumor cell and cell-free RNA capture and expression analysis identify platelet-associated genes in metastatic Lung cancer. BMC Cancer 19(1):603. https://doi.org/10.1186/s12885-019-5795-x
Cui HY, Wang SJ, Song F, Cheng X, Nan G, Zhao Y, Qian MR, Chen X, Li JY, Liu FL, Zhu YM, Tian RF, Wang B, Wu B, Zhang Y, Sun XX, Guo T, Yang XM, Zhang H, Li L, Xu J, Bian HJ, Jiang JL, Chen ZN (2021) CD147 receptor is essential for TFF3-mediated signaling regulating Colorectal cancer progression. Signal Transduct Target Ther 6(1):268. https://doi.org/10.1038/s41392-021-00677-2
Dai Y, Pierson SE, Dudney WC, Stack BC Jr (2010) Extraribosomal function of metallopanstimulin-1: reducing paxillin in head and neck squamous cell carcinoma and inhibiting Tumor growth. Int J Cancer 126(3):611–619. https://doi.org/10.1002/ijc.24791
**ao Y, Ma J, Guo C, Liu D, Pan J, Huang X (2022) Cyclin B2 overexpression promotes tumour growth by regulating jagged 1 in hepatocellular carcinoma. Aging 14(6):2855–2867. https://doi.org/10.18632/aging.203979
Dasgupta P, Kulkarni P, Majid S, Hashimoto Y, Shiina M, Shahryari V, Bhat NS, Tabatabai L, Yamamura S, Saini S, Tanaka Y, Dahiya R (2020) LncRNA CDKN2B-AS1/miR-141/cyclin D network regulates Tumor progression and Metastasis of renal cell carcinoma. Cell Death Dis 11(8):660. https://doi.org/10.1038/s41419-020-02877-0
Godoy PRDV, Donaires FS, Montaldi APL, Sakamoto-Hojo ET (2021) Anti-proliferative effects of E2F1 suppression in Glioblastoma Cells. Cytogenet Genome Res 161(6–7):372–381. https://doi.org/10.1159/000516997
Naderi A (2019) Molecular functions of brain expressed X-linked 2 (BEX2) in malignancies. Exp Cell Res 376(2):221–226. https://doi.org/10.1016/j.yexcr.2019.02.014
Ji H, Liu N, Yin Y, Wang X, Chen X, Li J, Li J (2018) Oxytocin inhibits Ovarian cancer Metastasis by repressing the expression of MMP-2 and VEGF. J Cancer 9(8):1379–1384. https://doi.org/10.7150/jca.23769
Sánchez ML, Coveñas R (2022) The galaninergic system: a target for cancer treatment. Cancers https://doi.org/10.3390/cancers14153755
Ji R, Ji Y, Ma L, Ge S, Chen J, Wu S, Huang T, Sheng Y, Wang L, Yi N, Liu Z (2021) Keratin 17 upregulation promotes cell Metastasis and angiogenesis in colon adenocarcinoma. Bioengineered 12(2):12598–12611. https://doi.org/10.1080/21655979.2021.2010393
Yang L, Zhang S, Wang G (2019) Keratin 17 in Disease pathogenesis: from cancer to dermatoses. J Pathol 247(2):158–165. https://doi.org/10.1002/path.5178
Kang HG, Kim WJ, Noh MG, Chun KH, Kim SJ (2020) SPON2 Is upregulated through notch signaling pathway and promotes tumor progression in gastric cancer. Cancers https://doi.org/10.3390/cancers12061439
Lv L, Liu FR, Na D, Xu HM, Wang ZN, Jiang CG (2020) Transforming growth factor-β1 induces connective tissue growth factor expression and promotes peritoneal metastasis of gastric cancer. Biosci Rep. https://doi.org/10.1042/bsr20201501
Pouliot N, Kusuma N (2013) Laminin-511: a multi-functional adhesion protein regulating cell migration, Tumor invasion and Metastasis. Cell Adh Migr 7(1):142–149. https://doi.org/10.4161/cam.22125
Frazzi R (2021) BIRC3 and BIRC5: multi-faceted inhibitors in cancer. Cell Biosci 11(1):8. https://doi.org/10.1186/s13578-020-00521-0
Han ZW, Lyv ZW, Cui B, Wang YY, Cheng JT, Zhang Y, Cai WQ, Zhou Y, Ma ZW, Wang XW, Peng XC, Cui SZ, **ang Y, Yang M, **n HW (2020) Correction to: the old CEACAMs find their new role in Tumor immunotherapy. Invest New Drugs 38(6):1899–1900. https://doi.org/10.1007/s10637-020-00967-6
Gu S, Zaidi S, Hassan MI, Mohammad T, Malta TM, Noushmehr H, Nguyen B, Crandall KA, Srivastav J, Obias V, Lin P, Nguyen BN, Yao M, Yao R, King CH, Mazumder R, Mishra B, Rao S, Mishra L (2020) Mutated CEACAMs disrupt transforming growth factor Beta Signaling and alter the intestinal microbiome to promote colorectal carcinogenesis. Gastroenterology 158(1):238–252. https://doi.org/10.1053/j.gastro.2019.09.023
Hu T, Liu H, Liang Z, Wang F, Zhou C, Zheng X, Zhang Y, Song Y, Hu J, He X, **ao J, King RJ, Wu X, Lan P (2020) Tumor-intrinsic CD47 signal regulates glycolysis and promotes Colorectal cancer cell growth and Metastasis. Theranostics 10(9):4056–4072. https://doi.org/10.7150/thno.40860
Nogués L, Palacios-García J, Reglero C, Rivas V, Neves M, Ribas C, Penela P, Mayor F (2018) G protein-coupled receptor kinases (GRKs) in tumorigenesis and cancer progression: GPCR regulators and signaling hubs. Semin Cancer Biol 48:78–90. https://doi.org/10.1016/j.semcancer.2017.04.013
Venuto S, Coda ARD, González-Pérez R, Laselva O, Tolomeo D, Storlazzi CT, Liso A, Conese M (2023) IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression. Int J Mol Sci. https://doi.org/10.3390/ijms24054804
Massagué J, Ganesh K (2021) Metastasis-initiating cells and ecosystems. Cancer Discov 11(4):971–994. https://doi.org/10.1158/2159-8290.Cd-21-0010
Wang A, Chen L, Li C, Zhu Y (2015) Heterogeneity in cancer stem cells. Cancer Lett 357(1):63–68. https://doi.org/10.1016/j.canlet.2014.11.040
Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK (2020) EMT, MET, plasticity, and Tumor Metastasis. Trends Cell Biol 30(10):764–776. https://doi.org/10.1016/j.tcb.2020.07.003
Wu M, Zhang X, Zhang W, Chiou YS, Qian W, Liu X, Zhang M, Yan H, Li S, Li T, Han X, Qian P, Liu S, Pan Y, Lobie PE, Zhu T (2022) Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat Commun 13(1):1371. https://doi.org/10.1038/s41467-022-29018-9
Bado IL, Zhang W, Hu J, Xu Z, Wang H, Sarkar P, Li L, Wan YW, Liu J, Wu W, Lo HC, Kim IS, Singh S, Janghorban M, Muscarella AM, Goldstein A, Singh P, Jeong HH, Liu C, Schiff R, Huang S, Ellis MJ, Gaber MW, Gugala Z, Liu Z, Zhang XH (2021) The bone microenvironment increases phenotypic plasticity of ER(+) Breast cancer cells. Dev Cell 56(8):1100–1117e1109. https://doi.org/10.1016/j.devcel.2021.03.008
Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, Thiru P, Bierie B, Ye X, Burge CB, Weinberg RA (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal Breast cancer cells. Proc Natl Acad Sci 116(15):7353–7362. https://doi.org/10.1073/pnas.1812876116
Koizumi M, Hiasa Y, Kumagi T, Yamanishi H, Azemoto N, Kobata T, Matsuura B, Abe M, Onji M (2013) Increased B cell-activating factor promotes Tumor invasion and Metastasis in human Pancreatic cancer. PLoS ONE 8(8):e71367. https://doi.org/10.1371/journal.pone.0071367
Yuan S, Norgard RJ, Stanger BZ (2019) Cellular Plasticity in Cancer. Cancer Discov 9(7):837–851. https://doi.org/10.1158/2159-8290.Cd-19-0015
Boumahdi S, de Sauvage FJ (2020) The great Escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov 19(1):39–56. https://doi.org/10.1038/s41573-019-0044-1
Cho ES, Kang HE, Kim NH, Yook JI (2019) Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharmacal Res 42(1):14–24. https://doi.org/10.1007/s12272-018-01108-7
Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, Zhu F, Zhou D, Zheng S, Chen Y, Zhou J (2021) Circulating Tumor cells: biology and clinical significance. Signal Transduct Target Ther 6(1):404. https://doi.org/10.1038/s41392-021-00817-8
Pantel K, Speicher MR (2016) The biology of circulating Tumor cells. Oncogene 35(10):1216–1224. https://doi.org/10.1038/onc.2015.192
Liu X, Li J, Cadilha BL, Markota A, Voigt C, Huang Z, Lin PP, Wang DD, Dai J, Kranz G, Krandick A, Libl D, Zitzelsberger H, Zagorski I, Braselmann H, Pan M, Zhu S, Huang Y, Niedermeyer S, Reichel CA, Uhl B, Briukhovetska D, Suárez J, Kobold S, Gires O, Wang H (2019) Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of Metastasis. Sci Adv 5(6):eaav4275. https://doi.org/10.1126/sciadv.aav4275
Schuster E, Taftaf R, Reduzzi C, Albert MK, Romero-Calvo I, Liu H (2021) Better together: circulating Tumor cell clustering in metastatic cancer. Trends Cancer 7(11):1020–1032. https://doi.org/10.1016/j.trecan.2021.07.001
Ganesh K, Basnet H, Kaygusuz Y, Laughney AM, He L, Sharma R, O’Rourke KP, Reuter VP, Huang YH, Turkekul M, Er EE, Masilionis I, Manova-Todorova K, Weiser MR, Saltz LB, Garcia-Aguilar J, Koche R, Lowe SW, Pe’er D, Shia J, Massagué J (2020) L1CAM defines the regenerative origin of metastasis-initiating cells in Colorectal cancer. Nat Cancer 1(1):28–45. https://doi.org/10.1038/s43018-019-0006-x
Piñeiro R, Martínez-Pena I, López-López R (2020) Relevance of CTC clusters in Breast Cancer Metastasis. Adv Exp Med Biol 1220:93–115. https://doi.org/10.1007/978-3-030-35805-1_7
Schlesinger M (2018) Role of platelets and platelet receptors in cancer Metastasis. J Hematol Oncol 11(1):125. https://doi.org/10.1186/s13045-018-0669-2
Liu Y, Zhang Y, Ding Y, Zhuang R (2021) Platelet-mediated Tumor Metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol 167:103502. https://doi.org/10.1016/j.critrevonc.2021.103502
Ren J, He J, Zhang H, **a Y, Hu Z, Loughran P, Billiar T, Huang H, Tsung A (2021) Platelet TLR4-ERK5 Axis facilitates NET-Mediated capturing of circulating Tumor cells and distant Metastasis after Surgical stress. Cancer Res 81(9):2373–2385. https://doi.org/10.1158/0008-5472.Can-20-3222
Tao DL, Tassi Yunga S, Williams CD, McCarty OJT (2021) Aspirin and antiplatelet treatments in cancer. Blood 137(23):3201–3211. https://doi.org/10.1182/blood.2019003977
Haider T, Tiwari R, Vyas SP, Soni V (2019) Molecular determinants as therapeutic targets in cancer chemotherapy: an update. Pharmacol Ther 200:85–109. https://doi.org/10.1016/j.pharmthera.2019.04.011
Vimalraj S (2022) A concise review of VEGF, PDGF, FGF, notch, angiopoietin, and HGF signalling in Tumor angiogenesis with a focus on alternative approaches and future directions. Int J Biol Macromol 221:1428–1438. https://doi.org/10.1016/j.ijbiomac.2022.09.129
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes Metastasis. Cancer Cell 20(5):576–590. https://doi.org/10.1016/j.ccr.2011.09.009
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ (2019) E-cadherin is required for Metastasis in multiple models of Breast cancer. Nature 573(7774):439–444. https://doi.org/10.1038/s41586-019-1526-3
Naxerova K (2020) Defining the role of lymph node Metastasis in systemic Breast cancer evolution. EBioMedicine 57:102852. https://doi.org/10.1016/j.ebiom.2020.102852
Tjan-Heijnen V, Viale G (2018) The Lymph Node and the Metastasis. N Engl J Med 378(21):2045–2046. https://doi.org/10.1056/NEJMcibr1803854
Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin SM, Kitahara S, Bouta EM, Chang J, Beech E, Jeong HS, Carroll MC, Taghian AG, Padera TP (2018) Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359(6382):1403–1407. https://doi.org/10.1126/science.aal3622
Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M, Kerjaschki D (2018) Lymph node blood vessels provide exit routes for metastatic Tumor cell dissemination in mice. Science 359(6382):1408–1411. https://doi.org/10.1126/science.aal3662
Jones D, Pereira ER, Padera TP (2018) Growth and Immune Evasion of Lymph Node Metastasis. Front Oncol 8:36. https://doi.org/10.3389/fonc.2018.00036
Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR, Zhao Z, Mathews TP, Morrison SJ (2020) Lymph protects metastasizing Melanoma cells from ferroptosis. Nature 585(7823):113–118. https://doi.org/10.1038/s41586-020-2623-z
Pascual-Antón L, Cardeñes B, de la Sainz R, González-Cortijo L, López-Cabrera M, Cabañas C, Sandoval P (2021) Mesothelial-to-mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. Int J Mol Sci. https://doi.org/10.3390/ijms222111496
Lugassy C, Kleinman HK, Vermeulen PB, Barnhill RL (2020) Angiotropism, pericytic mimicry and extravascular migratory Metastasis: an embryogenesis-derived program of Tumor spread. Angiogenesis 23(1):27–41. https://doi.org/10.1007/s10456-019-09695-9
Abdelaziz TT, Abdel Razek AAK (2022) Magnetic resonance imaging of Perineural Spread of Head and Neck Cancer. Magn Reson Imaging Clin N Am 30(1):95–108. https://doi.org/10.1016/j.mric.2021.06.017
Kim M, Hwang J, Kim KA, Hwang S, Lee HJ, Jung JY, Lee JG, Cha YJ, Shim HS (2022) Genomic characteristics of invasive mucinous adenocarcinoma of the lung with multiple pulmonary sites of involvement. Mod Pathol 35(2):202–209. https://doi.org/10.1038/s41379-021-00872-0
**ao Y, Yu D (2021) Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753
Wei J, Hu M, Huang K, Lin S, Du H (2020) Roles of proteoglycans and glycosaminoglycans in cancer development and progression. Int J Mol Sci. https://doi.org/10.3390/ijms21175983
Sari A, Cyr DP, Brar A, Messenger DE, Driman DK, Shivji S, Assarzadegan N, Juda A, Swallow CJ, Kennedy ED, Brar MS, Conner J, Kirsch R (2022) Routine elastin staining in surgically resected Colorectal Cancer: impact on Venous Invasion Detection and its Association with oncologic outcomes. Am J Surg Pathol 46(2):200–212. https://doi.org/10.1097/pas.0000000000001790
Kaur A, Ecker BL, Douglass SM, Kugel CH 3rd, Webster MR, Almeida FV, Somasundaram R, Hayden J, Ban E, Ahmadzadeh H, Franco-Barraza J, Shah N, Mellis IA, Keeney F, Kossenkov A, Tang HY, Yin X, Liu Q, Xu X, Fane M, Brafford P, Herlyn M, Speicher DW, Wargo JA, Tetzlaff MT, Haydu LE, Raj A, Shenoy V, Cukierman E, Weeraratna AT (2019) Remodeling of the Collagen Matrix in aging skin promotes Melanoma Metastasis and affects Immune Cell Motility. Cancer Discov 9(1):64–81. https://doi.org/10.1158/2159-8290.Cd-18-0193
Fidler IJ (2003) The pathogenesis of cancer Metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3(6):453–458. https://doi.org/10.1038/nrc1098
Hernandez L, Smirnova T, Kedrin D, Wyckoff J, Zhu L, Stanley ER, Cox D, Muller WJ, Pollard JW, Van Rooijen N, Segall JE (2009) The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta1 and CXCL12. Cancer Res 69(7):3221–3227. https://doi.org/10.1158/0008-5472.Can-08-2871
Li B, Liu S, Yang Q, Li Z, Li J, Wu J, Sun S, Xu Z, Sun S, Wu Q (2023) Macrophages in Tumor-Associated Adipose Microenvironment accelerate Tumor Progression. Adv Biol (Weinh) 7(1):e2200161. https://doi.org/10.1002/adbi.202200161
Xu M, Zhang T, **a R, Wei Y, Wei X (2022) Targeting the Tumor stroma for cancer therapy. Mol Cancer 21(1):208. https://doi.org/10.1186/s12943-022-01670-1
Haider T, Pandey V, Banjare N, Gupta PN, Soni V (2020) Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol Rep 72(5):1125–1151. https://doi.org/10.1007/s43440-020-00138-7
Manore SG, Doheny DL, Wong GL, Lo HW (2022) IL-6/JAK/STAT3 signaling in Breast Cancer Metastasis: Biology and Treatment. Front Oncol 12:866014. https://doi.org/10.3389/fonc.2022.866014
Morin PJ (2003) Drug resistance and the microenvironment: nature and nurture. Drug Resist Updates 6(4):169–172. https://doi.org/10.1016/s1368-7646(03)00059-1
**a T, Li J, Ren X, Liu C, Sun C (2021) Research progress of phenolic compounds regulating IL-6 to exert antitumor effects. Phytother Res 35(12):6720–6734. https://doi.org/10.1002/ptr.7258
Feng Y, Yang Z, Xu X (2022) c-Met: a promising therapeutic target in Bladder Cancer. Cancer Manag Res 14:2379–2388. https://doi.org/10.2147/cmar.S369175
Boromand N, Hasanzadeh M, ShahidSales S, Farazestanian M, Gharib M, Fiuji H, Behboodi N, Ghobadi N, Hassanian SM, Ferns GA, Avan A (2018) Clinical and prognostic value of the C-Met/HGF signaling pathway in Cervical cancer. J Cell Physiol 233(6):4490–4496. https://doi.org/10.1002/jcp.26232
Qin T, **ao Y, Qian W, Wang X, Gong M, Wang Q, An R, Han L, Duan W, Ma Q, Wang Z (2022) HGF/c-Met pathway facilitates the perineural invasion of Pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis 13(4):387. https://doi.org/10.1038/s41419-022-04799-5
Zhao J, **ao A, Liu C, Ye C, Yin K, Lu M, Jiao R, Chen X, Zhang C, Liu M (2020) The HIF-1A/miR-17-5p/PDCD4 axis contributes to the Tumor growth and Metastasis of gastric cancer. Signal Transduct Target Ther 5(1):46. https://doi.org/10.1038/s41392-020-0132-z
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, Liao WT, Ding YQ, Liang L (2019) CAFs secreted exosomes promote Metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in Colorectal cancer. Mol Cancer 18(1):91. https://doi.org/10.1186/s12943-019-1019-x
Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W, Liang Y, Zhang N, Yang Q (2021) Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes Breast cancer cell proliferation and Metastasis through targeting USP28. Theranostics 11(8):3932–3947. https://doi.org/10.7150/thno.53412
Akhtar M, Haider A, Rashid S, Al-Nabet A (2019) Paget’s seed and soil theory of Cancer Metastasis: an idea whose time has come. Adv Anat Pathol 26(1):69–74. https://doi.org/10.1097/pap.0000000000000219
Fornetti J, Welm AL, Stewart SA (2018) Understanding the bone in Cancer Metastasis. J Bone Miner Res 33(12):2099–2113. https://doi.org/10.1002/jbmr.3618
Gong Z, Li Q, Shi J, Wei J, Li P, Chang CH, Shultz LD, Ren G (2022) Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 55(8):1483–1500e1489. https://doi.org/10.1016/j.immuni.2022.07.001
Catena R, Bhattacharya N, El Rayes T, Wang S, Choi H, Gao D, Ryu S, Joshi N, Bielenberg D, Lee SB, Haukaas SA, Gravdal K, Halvorsen OJ, Akslen LA, Watnick RS, Mittal V (2013) Bone marrow-derived Gr1 + cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov 3(5):578–589. https://doi.org/10.1158/2159-8290.Cd-12-0476
Farolfi A, Altavilla A, Morandi L, Capelli L, Chiadini E, Prisinzano G, Gurioli G, Molari M, Calistri D, Foschini MP, De Giorgi U (2022) Endometrioid Cancer Associated with endometriosis: from the seed and soil theory to clinical practice. Front Oncol 12:859510. https://doi.org/10.3389/fonc.2022.859510
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF (2017) 20-Year risks of Breast-Cancer recurrence after stop** endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846. https://doi.org/10.1056/NEJMoa1701830
Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA (2020) The current paradigm and challenges ahead for the dormancy of disseminated Tumor cells. Nat Cancer 1(7):672–680. https://doi.org/10.1038/s43018-020-0088-5
Malladi S, Macalinao DG, ** X, He L, Basnet H, Zou Y, de Stanchina E, Massagué J (2016) Metastatic latency and Immune Evasion through Autocrine inhibition of WNT. Cell 165(1):45–60. https://doi.org/10.1016/j.cell.2016.02.025
Hadfield G (1954) The dormant cancer cell. Br Med J 2(4888):607–610. https://doi.org/10.1136/bmj.2.4888.607
Phan TG, Croucher PI (2020) The dormant cancer cell life cycle. Nat Rev Cancer 20(7):398–411. https://doi.org/10.1038/s41568-020-0263-0
Shen S, Vagner S, Robert C (2020) Persistent Cancer cells: the Deadly survivors. Cell 183(4):860–874. https://doi.org/10.1016/j.cell.2020.10.027
Gomatou G, Syrigos N, Vathiotis IA, Kotteas EA (2021) Tumor dormancy: implications for invasion and metastasis. Int J Mol Sci. https://doi.org/10.3390/ijms22094862
Recasens A, Munoz L (2019) Targeting Cancer Cell Dormancy. Trends Pharmacol Sci 40(2):128–141. https://doi.org/10.1016/j.tips.2018.12.004
Jiang J, Zheng M, Zhang M, Yang X, Li L, Wang SS, Wu JS, Yu XH, Wu JB, Pang X, Tang YJ, Tang YL, Liang XH (2019) PRRX1 regulates Cellular phenotype plasticity and dormancy of Head and Neck squamous cell carcinoma through miR-642b-3p. Neoplasia 21(2):216–229. https://doi.org/10.1016/j.neo.2018.12.001
Naumov GN, Akslen LA, Folkman J (2006) Role of angiogenesis in human Tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5(16):1779–1787. https://doi.org/10.4161/cc.5.16.3018
Zhao L, Lei J, Gu S, Zhang Y, **g X, Wang L, Zhang L, Ning Q, Luo M, Qi Y, Zhao X, Shao S (2022) A yes-associated protein 1- Notch1 receptor positive feedback loop promotes Breast cancer lung Metastasis by attenuating the bone morphogenetic protein 4-SMAD family member 1/5 signaling. Carcinogenesis 43(12):1162–1175. https://doi.org/10.1093/carcin/bgac081
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, Giancotti FG, Schachner M, Malladi S, Massagué J (2018) Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol 20(8):966–978. https://doi.org/10.1038/s41556-018-0138-8
De Cock JM, Shibue T, Dongre A, Keckesova Z, Reinhardt F, Weinberg RA (2016) Inflammation triggers Zeb1-Dependent Escape from Tumor latency. Cancer Res 76(23):6778–6784. https://doi.org/10.1158/0008-5472.Can-16-0608
Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. https://doi.org/10.1126/science.aao4227
Sakaguchi T, Valente R, Tanaka K, Satoi S, Del Chiaro M (2019) Surgical treatment of metastatic pancreatic ductal adenocarcinoma: a review of current literature. Pancreatology 19(5):672–680. https://doi.org/10.1016/j.pan.2019.05.466
Rocha B, de Morais LA, Viana MC, Carneiro G (2023) Promising strategies for improving oral bioavailability of poor water-soluble Drugs. Expert Opin Drug Discov 18(6):615–627. https://doi.org/10.1080/17460441.2023.2211801
Annaji M, Poudel I, Boddu SHS, Arnold RD, Tiwari AK, Babu RJ (2021) Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep (Hoboken) 4(3):e1353. https://doi.org/10.1002/cnr2.1353
Pandey P, Gulati N, Makhija M, Purohit D, Dureja H (2020) Nanoemulsion: a Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat Nanotechnol 14(4):276–293. https://doi.org/10.2174/1872210514666200604145755
Khan KU, Minhas MU, Badshah SF, Suhail M, Ahmad A, Ijaz S (2022) Overview of nanoparticulate strategies for solubility enhancement of poorly soluble Drugs. Life Sci 291:120301. https://doi.org/10.1016/j.lfs.2022.120301
Maeda H (2012) Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Controlled Release 164(2):138–144. https://doi.org/10.1016/j.jconrel.2012.04.038
Maeda H (2010) Tumor-selective delivery of macromolecular Drugs via the EPR Effect: background and future prospects. Bioconjug Chem 21(5):797–802. https://doi.org/10.1021/bc100070g
Spleis H, Sandmeier M, Claus V, Bernkop-Schnürch A (2023) Surface design of nanocarriers: key to more efficient oral drug delivery systems. Adv Colloid Interface Sci 313:102848. https://doi.org/10.1016/j.cis.2023.102848
Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, Dobrovolskaia MA (2022) To PEGylate or not to PEGylate: immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev 180:114079. https://doi.org/10.1016/j.addr.2021.114079
Gigmes D, Trimaille T (2021) Advances in amphiphilic polylactide/vinyl polymer based nano-assemblies for drug delivery. Adv Colloid Interface Sci 294:102483. https://doi.org/10.1016/j.cis.2021.102483
Koeppe H, Horn D, Scholz J, Quaas E, Schötz S, Reisbeck F, Achazi K, Mohammadifar E, Dernedde J, Haag R (2023) Shell-sheddable dendritic polyglycerol sulfates loaded with sunitinib for inhibition of Tumor angiogenesis. Int J Pharm 642:123158. https://doi.org/10.1016/j.ijpharm.2023.123158
Liu G, Chen T, Ding Z, Wang Y, Wei Y, Wei X (2021) Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment. Cell Prolif 54(4):e13009. https://doi.org/10.1111/cpr.13009
Guo Z, He B, Yuan L, Dai W, Zhang H, Wang X, Wang J, Zhang X, Zhang Q (2015) Dual targeting for metastatic Breast cancer and Tumor neovasculature by EphA2-mediated nanocarriers. Int J Pharm 493(1):380–389. https://doi.org/10.1016/j.ijpharm.2015.05.051
Yang X, Zhao J, Duan S, Hou X, Li X, Hu Z, Tang Z, Mo F, Lu X (2019) Enhanced cytotoxic T lymphocytes recruitment targeting Tumor vasculatures by endoglin aptamer and IP-10 plasmid presenting liposome-based nanocarriers. Theranostics 9(14):4066–4083. https://doi.org/10.7150/thno.33383
Yu X, Su Q, Chang X, Chen K, Yuan P, Liu T, Tian R, Bai Y, Zhang Y, Chen X (2021) Multimodal obstruction of tumorigenic energy supply via bionic nanocarriers for effective Tumor therapy. Biomaterials 278:121181. https://doi.org/10.1016/j.biomaterials.2021.121181
Zhou M, Yao Y, Ma S, Zou M, Chen Y, Cai S, Zhao F, Wu H, **ao F, Abudushalamu G, Fan X, Wu G (2023) Dual-targeted and dual-sensitive self-assembled protein nanocarrier delivering hVEGI-192 for triple-negative Breast cancer. Int J Biol Macromol 245:125475. https://doi.org/10.1016/j.ijbiomac.2023.125475
Zhong X, Chen B, Yang Z (2018) The role of Tumor-Associated macrophages in Colorectal Carcinoma Progression. Cell Physiol Biochem 45(1):356–365. https://doi.org/10.1159/000486816
Zhang H, Zhang X, Ren Y, Cao F, Hou L, Zhang Z (2019) An in situ microenvironmental nano-regulator to inhibit the proliferation and Metastasis of 4T1 Tumor. Theranostics 9(12):3580–3594. https://doi.org/10.7150/thno.33141
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT (2019) Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 10(1):3974. https://doi.org/10.1038/s41467-019-11911-5
Ye J, Yang Y, Dong W, Gao Y, Meng Y, Wang H, Li L, ** J, Ji M, **a X, Chen X, ** Y, Liu Y (2019) Drug-free mannosylated liposomes inhibit Tumor growth by promoting the polarization of tumor-associated macrophages. Int J Nanomed 14:3203–3220. https://doi.org/10.2147/ijn.S207589
Gu X, Zhang R, Sun Y, Ai X, Wang Y, Lyu Y, Wang X, Wu Y, Wang Z, Feng N, Liu Y (2023) Oral membrane-biomimetic nanoparticles for enhanced endocytosis and regulation of tumor-associated macrophage. J Nanobiotechnol 21(1):206. https://doi.org/10.1186/s12951-023-01949-5
Li X, Ji Q, Yan C, Zhu Z, Yan Z, Chen P, Wang Y, Song L (2022) H(2)O(2)/pH dual-responsive Biomimetic Nanoenzyme Drugs Delivery System for enhanced Tumor photodynamic therapy. Nanoscale Res Lett 17(1):103. https://doi.org/10.1186/s11671-022-03738-9
Sun Y, Zhao D, Wang G, Wang Y, Cao L, Sun J, Jiang Q, He Z (2020) Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta Pharm Sin B 10(8):1382–1396. https://doi.org/10.1016/j.apsb.2020.01.004
Matuszewska K, Pereira M, Petrik D, Lawler J, Petrik J (2021) Normalizing tumor vasculature to reduce hypoxia, enhance perfusion, and optimize therapy uptake. Cancers. https://doi.org/10.3390/cancers13174444
Feng Q, Li Y, Yang X, Zhang W, Hao Y, Zhang H, Hou L, Zhang Z (2018) Hypoxia-specific therapeutic agents delivery nanotheranostics: a sequential strategy for ultrasound mediated on-demand tritherapies and imaging of cancer. J Controlled Release 275:192–200. https://doi.org/10.1016/j.jconrel.2018.02.011
Yin W, Qiang M, Ke W, Han Y, Mukerabigwi JF, Ge Z (2018) Hypoxia-responsive block copolymer radiosensitizers as anticancer drug nanocarriers for enhanced chemoradiotherapy of bulky solid tumors. Biomaterials 181:360–371. https://doi.org/10.1016/j.biomaterials.2018.08.014
Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, Jo DG, Ahn CH, Suh YD, Kim K, Kwon IC, Lee DS, Park JH (2014) Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 35(5):1735–1743. https://doi.org/10.1016/j.biomaterials.2013.11.022
Thambi T, Son S, Lee DS, Park JH (2016) Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater 29:261–270. https://doi.org/10.1016/j.actbio.2015.10.011
Ahmad Z, Lv S, Tang Z, Shah A, Chen X (2016) Methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for potential hypoxia-responsive delivery of doxorubicin. J Biomater Sci Polym Ed 27(1):40–54. https://doi.org/10.1080/09205063.2015.1107707
Zhou X, You M, Wang F, Wang Z, Gao X, **g C, Liu J, Guo M, Li J, Luo A, Liu H, Liu Z, Chen C (2021) Multifunctional graphdiyne-cerium Oxide Nanozymes facilitate MicroRNA delivery and attenuate Tumor Hypoxia for highly efficient Radiotherapy of Esophageal Cancer. Adv Mater 33(24):e2100556. https://doi.org/10.1002/adma.202100556
Chen X, Li Q, Huang Z, Lin W, Ma Y (2022) Construction and evaluation of curcumin upconversion nanocarriers decorated with MnO(2) for Tumor photodynamic therapy. Drug Deliv Transl Res 12(11):2678–2692. https://doi.org/10.1007/s13346-022-01118-5
Li Z, Wang F, Li Y, Wang X, Lu Q, Wang D, Qi C, Li C, Li Z, Lian B, Tian G, Gao Z, Zhang B, Wu J (2021) Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and Metastasis via targeting substance P-hepatic stellate cells-hepatocellular carcinoma axis. Biomaterials 276:121003. https://doi.org/10.1016/j.biomaterials.2021.121003
González-Sarrías A, Iglesias-Aguirre CE, Cortés-Martín A, Vallejo F, Cattivelli A, Del Pozo-Acebo L, Del Saz A, López de Las Hazas MC, Dávalos A, Espín JC (2022) Milk-derived exosomes as nanocarriers to deliver curcumin and resveratrol in breast tissue and enhance their anticancer activity. Int J Mol Sci. https://doi.org/10.3390/ijms23052860
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in Pancreatic cancer. Nature 546(7659):498–503. https://doi.org/10.1038/nature22341
Gong Z, Liu X, Zhou B, Wang G, Guan X, Xu Y, Zhang J, Hong Z, Cao J, Sun X, Gao Z, Lu H, Pan X, Bai J (2021) Tumor acidic microenvironment-induced drug release of RGD peptide nanoparticles for cellular uptake and cancer therapy. Colloids Surf B 202:111673. https://doi.org/10.1016/j.colsurfb.2021.111673
Wong-Rolle A, Wei HK, Zhao C, ** C (2021) Unexpected guests in the Tumor microenvironment: microbiome in cancer. Protein Cell 12(5):426–435. https://doi.org/10.1007/s13238-020-00813-8
Gong Z, Liu X, Dong J, Zhang W, Jiang Y, Zhang J, Feng W, Chen K, Bai J (2019) Transition from vesicles to nanofibres in the enzymatic self-assemblies of an amphiphilic peptide as an antitumour drug carrier. Nanoscale 11(33):15479–15486. https://doi.org/10.1039/c9nr02874a
Gong Z, Liu X, Wu J, Li X, Tang Z, Deng Y, Sun X, Chen K, Gao Z, Bai J (2020) pH-triggered morphological change in a self-assembling amphiphilic peptide used as an antitumor drug carrier. Nanotechnology 31(16):165601. https://doi.org/10.1088/1361-6528/ab667c
Hong Z, Sun X, Sun X, Cao J, Yang Z, Pan Z, Yu T, Dong J, Zhou B, Bai J (2021) Enzyme-induced morphological transformation of drug carriers: implications for cytotoxicity and the retention time of antitumor agents. Mater Sci Engineering: C 129:112389. https://doi.org/10.1016/j.msec.2021.112389
Cao J, Yuan X, Sun X, Meng F, Li H, Hong Z, Liu Y, Zhai X, Ma J, Peng S, Zhou Y, Liu X, Hao J, Bai J (2023) Matrix Metalloproteinase-2-Induced Morphologic Transformation of Self-assembled peptide Nanocarriers inhibits Tumor Growth and Metastasis. ACS Mater Lett 5(3):900–908. https://doi.org/10.1021/acsmaterialslett.2c01093
Cao J, Liu X, Yuan X, Meng F, Sun X, Xu L, Li H, Liu Y, Hong Z, Bai J (2023) Enzyme-induced morphological transformation of self-assembled peptide nanovehicles potentiates intratumoral aggregation and inhibits tumour immunosuppression. Chem Eng J 454:140466. https://doi.org/10.1016/j.cej.2022.140466
Peng S, Yuan X, Li H, Wei Y, Zhou B, Ding G, Bai J (2023) Recent progress in nanocarrier-based drug delivery systems for antitumour Metastasis. Eur J Med Chem 252:115259. https://doi.org/10.1016/j.ejmech.2023.115259
Kim K, Choi H, Choi ES, Park MH, Ryu JH (2019) Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics. https://doi.org/10.3390/pharmaceutics11070301
Song MJ, Liang Y, Li KK, Zhang J, Zhang N, Tian BC, Han JT (2019) Hyaluronic acid modified liposomes for targeted delivery of doxorubicin and paclitaxel to CD44 overexpressing tumor cells with improved dual-drugs synergistic effect. J Drug Delivery Sci Technol. https://doi.org/10.1016/j.jddst.2019.101179
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV (2018) Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine 14(1):195–204. https://doi.org/10.1016/j.nano.2017.09.011
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4):274–284. https://doi.org/10.1038/nrc2622
**a J, Ma S, Zhu X, Chen C, Zhang R, Cao Z, Chen X, Zhang L, Zhu Y, Zhang S, Li S, Gu G, Wei X, Yu K, Wang J (2022) Versatile ginsenoside Rg3 liposomes inhibit Tumor Metastasis by capturing circulating Tumor cells and destroying metastatic niches. Sci Adv 8(6):eabj1262. https://doi.org/10.1126/sciadv.abj1262
Yang X, Lian K, Tan Y, Zhu Y, Liu X, Zeng Y, Yu T, Meng T, Yuan H, Hu F (2020) Selective uptake of chitosan polymeric micelles by circulating monocytes for enhanced Tumor targeting. Carbohydr Polym 229:115435. https://doi.org/10.1016/j.carbpol.2019.115435
Wang Z, Chen C, Shi C, Zhao X, Gao L, Guo F, Han M, Yang Z, Zhang J, Tang C, Zhang C, Liu Y, Sun P, Jiang X (2023) Cell membrane derived liposomes loaded with DNase I target neutrophil extracellular traps which inhibits Colorectal cancer liver metastases. J Controlled Release 357:620–629. https://doi.org/10.1016/j.jconrel.2023.04.013
Xu XR, Yousef GM, Ni H (2018) Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood 131(16):1777–1789. https://doi.org/10.1182/blood-2017-05-743187
Li S, Li L, Lin X, Chen C, Luo C, Huang Y (2022) Targeted inhibition of Tumor inflammation and tumor-platelet crosstalk by nanoparticle-mediated drug delivery mitigates Cancer Metastasis. ACS Nano 16(1):50–67. https://doi.org/10.1021/acsnano.1c06022
Wang L, Liu G, Hu Y, Gou S, He T, Feng Q, Cai K (2022) Doxorubicin-loaded polypyrrole nanovesicles for suppressing Tumor Metastasis through combining photothermotherapy and lymphatic system-targeted chemotherapy. Nanoscale 14(8):3097–3111. https://doi.org/10.1039/D2NR00186A
Wang T-W, Yeh C-W, Kuan C-H, Wang L-W, Chen L-H, Wu H-C, Sun J-S (2017) Tailored design of multifunctional and programmable pH-responsive self-assembling polypeptides as drug delivery nanocarrier for cancer therapy. Acta Biomater 58:54–66. https://doi.org/10.1016/j.actbio.2017.06.008
Mao Y, Feng S, Li S, Zhao Q, Di D, Liu Y, Wang S (2019) Chylomicron-pretended nano-bio self-assembling vehicle to promote lymphatic transport and GALTs target of oral Drugs. Biomaterials 188:173–186. https://doi.org/10.1016/j.biomaterials.2018.10.012
Wang S, Wo L, Zhang Z, Zhu C, Wang C, Wang Y, Hou L, Cao H, Zhao Q, Zhao E (2022) Delivery of LINC00589 via mesoporous silica nanoparticles inhibits peritoneal Metastasis in gastric cancer. Cancer Lett 549:215916. https://doi.org/10.1016/j.canlet.2022.215916
Cabral H, Murakami M, Hojo H, Terada Y, Kano MR, Chung UI, Nishiyama N, Kataoka K (2013) Targeted therapy of spontaneous murine pancreatic tumors by polymeric micelles prolongs survival and prevents peritoneal Metastasis. Proc Natl Acad Sci 110(28):11397–11402. https://doi.org/10.1073/pnas.1301348110
Guo R, Deng M, He X, Li M, Li J, He P, Liu H, Li M, Zhang Z, He Q (2022) Fucoidan-functionalized activated platelet-hitchhiking micelles simultaneously track Tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharm Sin B 12(1):467–482. https://doi.org/10.1016/j.apsb.2021.05.012
Zhang J, Zuo T, Yang J, Hu Z, Wang Z, Xu R, Ma S, Wei Y, Shen Q (2021) Hierarchically releasing Bio-responsive nanoparticles for complete Tumor Microenvironment Modulation via TGF-β pathway inhibition and TAF reduction. ACS Appl Mater Interfaces 13(2):2256–2268. https://doi.org/10.1021/acsami.0c18545
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2023MC122) and the Open Projects Fund of NMPA Key Laboratory for Quality Research and Evaluation of Carbohydrate-based Medicine (2021QRECM03).
Author information
Authors and Affiliations
Contributions
HL: Methodology, software, writing—original draft. HH, HT, QJ and WS: Methodology, literature screening. QZ and BZ: Funding acquisition, supervision. JB: Conceptualization, writing—review & editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The research involved no human participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Huang, H., Tan, H. et al. Key processes in tumor metastasis and therapeutic strategies with nanocarriers: a review. Mol Biol Rep 51, 197 (2024). https://doi.org/10.1007/s11033-023-08910-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08910-7