Abstract
Idiopathic membranous nephropathy (IMN) belongs to an important pathogenic category of adult nephrotic syndrome. PLA2R1 exposure is critical for triggering the pathogenesis of PLA2R1-related IMN. However, the pathogenesis of IMN and the molecular mechanism of treatment remain to be further clarified. The expression changes of activated protein C (APC) and PLA2R1 in IMN patients were quantified by qPCR. A zymosan activated serum (ZAS)-induced IMN podocyte model was established in vitro. Podocyte apoptosis was detected via flow cytometry and caspase‑3 assay. The expression levels of APC, p-ERK1/2, ERK1/2, YB-1 and PLA2R1 were detected by western blotting. The regulation relationship between YB-1 and PLA2R1 was detected by dual fluorescent reporter system. In IMN patients, the expression level of PLA2R1 was increased, whereas the expression level of APC was decreased. When APC was added to podocytes in vitro, the phosphorylation of ERK1/2 was increased, which could promote the translocation of YB-1 to the nucleus that reduces the expression of PLA2R1 at the cellular transcriptional level, thereby inhibiting podocyte apoptosis. Our study is the first to report that APC can improve membranous nephropathy by affecting podocyte apoptosis through the ERK1/2/YB-1/PLA2R1 axis. This study will provide a new targeted therapy for IMN patients with high PLA2R1 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic membranous nephropathy (IMN) belongs to an important pathogenic category of adult nephrotic syndrome, caused by autoimmune reactions, accounting for about 25% to 40% of adult nephrotic syndrome [1,2,3]. The onset of idiopathic membranous nephropathy is slow and the clinical symptoms are different. The main manifestations are: massive proteinuria, hypoalbuminemia, hyperlipidemia, and hyperedema. About 1/3 of the patients can be complicated by deep vein thrombosis, low immune function, susceptible to infection. IMN is caused by podocyte-targeted IgG, of which IgG4 is thought to be the predominant form [3, 4]. Podocyte autoantigens recognized in adult IMN contain the M-type receptor for secreted phospholipase A2 (PLA2R1) [5], thrombospondin type 1 domain-containing 7A (THSD7A) [6], and neuroepidermal growth factor-like 1 protein (NELL1) [7]. In addition, the measurement of anti-PLA2R1 antibody in peripheral blood have been widely used as a biomarker for clinical diagnosis, prediction and treatment guidance [8]. Previous studies have shown that PLA2R1 exposure is essential for touching off the pathogenesis of PLA2R1-related IMN [9]. However, the pathogenesis of IMN and the molecular mechanism of treatment remain to be further clarified.
PLA2R1 is one of the mannose receptor family, which belongs to the type I transmembrane receptor and is localized on podocytes [10]. PLA2R1 is a specific receptor that facilitates the phospholipase A2 (sPLA2) internalization, and multiple research groups have found that the physiological role of PLA2R1 in the cells in which it is expressed is related to sPLA2s [11]. Interestingly, the oxidative extracellular milieu might cause PLA2R1 to establish or retain disulfide bonds, leading to long-term expression of pathogenic epitopes especially bound by antibodies against PLA2R1 circulate in peripheral blood of patients with IMN [9, 12]. Current study has shown that miR-130a-5p inhibits angiotensin II-induced podocyte apoptosis via regulating PLA2R1 [13]. PLA2R1 has been determined as the primary target antigen that triggers the cumulation of circulating autoantibodies in over 75% of IMN patients [5]. Previous findings have shown that reducing activation of PLA2R1 and inhibiting PI3K/AKT/mTOR signaling in PLA2R1-activated podocytes helped prevent podocyte apoptosis [14]. However, whether the podocyte membrane antigen PLA2R1 is regulated by other signaling pathways in the pathogenic autoimmune response is currently unclear.
Protein C is a plasma serine protease zymogen in its active form as activated protein C (APC) with great anticoagulant activity. APC exerts pleiotropic cytoprotective properties, including altered gene expression profiles, anti-inflammatory and anti-apoptotic effects, and stabilization of the endothelial and epithelial barrier [15, 16]. Recent studies have identified anti-inflammatory effects of APC in vascular endothelial cells, and APC is involved in regulatory crosstalk of EPCR, ERK, and NF-κB, thereby disrupting TNF signaling [17]. Further, previous data suggest that APC through PAR1/EPCR signaling maintain YB-1 levels by preventing YB-1 ubiquitination through OTUB1 [18]. Moreover, previous studies have shown that APC can improve diabetic nephropathy [19]. However, the molecular mechanism by which APC regulates YB-1 and whether it can improve IMN symptoms remains unknown.
Y-box-binding protein 1 (YB-1) is one of RNA-binding proteins involved in a variety of cellular processes such as RNA splicing, DNA damage repair and stress responses [20, 21]. Under healthy conditions, YB-1 is mainly found in the cytoplasm and plays a key role in regulating various aspects of RNA biology, but under conditions of genotoxic stress, YB-1 translocates to the nucleus, where activation affects immune response, apoptosis and tumor growth [20]. Previous study has shown that YB-1 can target the MEF2B promoter region to inhibit its expression and improve diabetic cardiomyopathy [18]. The role of YB-1 in calcineurin inhibitor-induced nephropathy was able to increase glomerular fibrosis but reduce interstitial fibrosis in the kidney [22]. Whether YB-1 regulates the expression of PLA2R1 in the ZAS-induced IMN model is still unknown.
Here, we reported the expression level of PLA2R1and APC in IMN patients. In our established ZAS-induced IMN cell model, the PLA2R1 expression was reduced through the ERK1/2/YB-1 pathway. Thereby inhibiting podocyte apoptosis. Therefore, our study is the first to report that APC can ameliorate membranous nephropathy by affecting podocyte apoptosis through the ERK1/2/YB-1/PLA2R1 axis.
Material and methods
Clinical specimens
All samples were obtained from patients at the Second Affiliated Hospital of Nanchang University with informed written consent and ethical approval granted by the Ethics Committee of the Second Affiliated Hospital of Nanchang University (Ethics approval number: [2019] No.111). The study was conducted in compliance with the International Ethical Guidelines for Research Involving Human Subjects of the Declaration of Helsinki. Kidney biopsies were performed with an 18-gauge crude needle to identify patients with primary membranous nephropathy by collecting samples from patients with positive anti-PLA2R1 antibodies in their blood. Normal kidney tissue from adult living kidney transplant donors was also collected as a control. Urine was collected from patients and normal people, and urine protein concentration was measured using a non-interfering protein assay kit (Calbiochem), and 24 h urine protein volume was calculated based on urine protein concentration and urine volume.
Cell culture
The human podocyte cell line was obtained from ATCC. Cells were grown in RPMI-1640 medium including 10% fetal bovine serum (Gibco), penicillin–streptomycin (Gibco), 1 mM l-glutamine and insulin, transferrin and selenium (Invitrogen) in 5% CO2 at 37 °C.
Cell treatment
To establish an in vitro podocyte model of IMN, Normal human serum was treated with Zymosan (Sigma) as previously described [23, 24]. Zymosan- activated human serum (ZAS) was used to assemble C5b-9. Human podocytes were treated for 1, 4, 8, 12, and 24 h in medium containing ZAS at a final concentration of 100 μL/mL, and heat-inactivated human serum (HIS) served as a control. For APC treatment experiments, human APC was purchased from Bei**g Aslaier Biotechnology Co., Ltd., and human podocytes were treated with different APC concentrations of 1 nM, 2 nM, 10 nM and 20 nM for 30 min. To inhibit ERK1/2 activity, podocytes were treated with SCH772984 (selleckchem), a specific inhibitor of ERK1/2.
Nucleus and cytoplasm separation experiment
Podocytes were harvested from cell culture dishes, rinsed in PBS and resuspended in hypotonic lysis buffer (10 mM HEPES pH 7.9; 1.5 mM MgCl2; 10 mM KCl; 0.5 mM dithiothreitol (DTT)) containing protease inhibitors. Cells were placed on ice for 30 min and then lysed through a 26-gauge needle. Optical microscopy confirmed that cell lysis reached 80–90% of the cell membrane was disrupted after centrifugation at 1,000 rcf for 5 min at 4 °C. The supernatant was collected and labeled as the cytoplasmic fraction. It was then washed once more in lysis buffer and resuspended in high-salt buffer (20 mM HEPES pH 7.9; 420 mM NaCl; 2 mM EDTA pH 8.0; 1.5 mM MgCl2; 25% glycerol; 0.5 mM DTT) containing protease inhibitors. After 30 min on ice, the pellet was centrifuged at 10,000 rcf at 4 °C. This nuclear pellet was again resuspended in high-salt buffer containing 1% Triton X-100 and 0.5% SDS, briefly sonicated, and representative of the nuclear fraction.
Plasmid construction
The knockdown shRNA of YB-1 was constructed into the pLKO.1 puro vector (addgene, 8453). For plasmids overexpressing YB-1 and PLA2R1, the cDNA sequences of human YB-1 and PLA2R1 were ligated into pCMV-M1 vectors (addgene, 23,007), respectively.
ELISA assay
The levels of PLA2R1 in the serum of patients with kidney disease were determined by enzyme-linked immunosorbent assay (ELISA) using reagents purchased from Wuhan Fine Biotech (EH2051) according to the experimental instructions. Briefly, patient serum samples were collected and added to a 96-well plate, then 100 μL of biotin antibody was added to each well and incubated for 1 h at 37 °C. Then 90 μL of TMB substrate was added to each well and incubated for 30 min at 37 °C protected from light. Finally, 50 μL of termination solution was added to each well and read immediately at 450 nm for analysis and statistics.
Immunofluorescence
Podocytes were fixed with 10% neutral buffered formalin, followed by blocking and permeabilization with 5% goat serum and 0.1% Triton X-100, and incubated overnight at 4 °C with antibodies against YB-1. DNA was then stained with 1 μg/mL 4',6-diamidino-2-phenylindole (DAPI). Goat anti-rabbit Alexa 488 (Thermo Fisher Scientific) was used as a secondary antibody. Observations were performed by LSM 810 confocal microscope (Zeiss, Germany).
Western blot
Podocytes were lysed and homogenized in RIPA buffer for 30 min on ice, then centrifuged at 12,000 rpm for 30 min at 4 °C. The supernatant was aspirated and added to 5 × protein loading buffer. The proteins were separated by SDS-PAGE gel electrophoresis and transferred to on a polyvinylidene fluoride membrane (Millipore). Membranes were blocked with 5% nonfat dry milk in TBST buffer for 1 h at room temperature and then incubated with primary antibody overnight at 4 °C. Blots were incubated with the corresponding primary antibodies. The primary antibodies used are as follows: APC antibody (ab40778, Abcam), PLA2R1 antibody (NBP1-84449, Novus Biologicals), β-Actin antibody (4967, Cell Signaling), p-ERK1/2 antibody (9101, Cell Signaling), ERK1/2 antibody (4695, Cell Signaling), YB-1 antibody (4202, Cell Signaling), Lamin B1 antibody (ab65986, Abcam). The next day was incubated with the corresponding secondary antibody. ECL exposure solution was used to visualize Blots (Thermo Fisher Scientific).
qPCR
Total RNA from podocytes was extracted with the MiRNeasy Mini Kit (Qiagen), followed by first-strand cDNA synthesis using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems). The ABI QuantStudio 5 (Applied Biosystems) system was used to measure the fluorescence intensity of real-time PCR reactions using Power-SYBR green PCR Master Mix (Applied Biosystems). Relative quantities of genes were computed by the 2–∆∆CT method and normalized to β-actin. The corresponding primers are shown below:
-
APC: F: 5’-AAGCATGAAACCGGCTCACAT-3’,
-
R: 5’-CATTCGTGTAGTTGAACCCTGA-3’;
-
YB-1: F: 5’-GGGGACAAGAAGGTCATCGC-3’,
-
R: 5’-CGAAGGTACTTCCTGGGGTTA-3’;
-
PLA2R1: F: 5’-GCATACAATCAAAGGGAACACCC-3’,
-
R: 5’-TCGTGGCACACCACAGTAAG-3’;
-
Β-actin: F: 5’-CTCCATCCTGGCCTCGCTGT-3’,
-
R: 5’-GCTGTCACCTTCACCGTTCC-3’.
Dual-luciferase activity assay
Cells were harvested 48 h after transfection. The experimental steps were performed according to the operating manual of the dual-luciferase reporter assay kit (Promega). The harvested cells were lysed on ice with lysis buffer, and the supernatant was collected by centrifugation at 4 °C for the next step of detection. Luciferase activity was detected using a luminometer (Promega). Renilla and firefly luciferase activities were measured in each sample and the ratio between them was calculated.
Flow cytometry
The ZAS-induced IMN model and the apoptosis of podocytes under different treatment conditions were detected. First, the podocytes grown in log phase were seeded in 6-well plates at 1 × 105 cells per well, and after culturing for 48 h, the podocytes were collected by trypsinization, washed three times with pre-cooled PBS, and then added with Annexin V-FITC binding solution. Resuspend cells gently, add Annexin V-FITC and propidium iodide staining solution, and mix gently. Finally, cells were incubated at room temperature in the dark for 20–30 min before analysis with a BD FACSLyric™ Clinical flow cytometer.
TUNEL assay
Cells were seeded onto 6-well plates, separated out, and cytospanned on slides. Cells were then fixed, permeabilized, and labeled according to the protocol of the TUNEL BrightRed Apoptosis Detection Kit (Vazyme). Apoptotic podocytes were determined by terminal deoxynucleotidyl transferase-mediated DUTP nick labeling (TUNEL). Nuclear counterstaining was performed with DAPI (Thermo Fisher Scientific). Images were captured under a fluorescent microscope. The ratio between apoptotic cells and nuclei was used to calculate the percentage of apoptotic cells.
Caspase‑3 assay
Podocytes were harvested and lysed with cell lysis buffer for 15 min on ice, followed by centrifugation at 20,000×g for 15 min at 4 °C. Transfer the supernatant to a pre-chilled centrifuge tube before assaying for caspase-3 activity. AC-DevD-PNA (2 mM; Beyotime) was added to the samples, followed by incubation at 37 °C for 2 h. To obtain the results, the absorbance value of each sample at 405 nm wavelength was measured using an enzyme label (ThermoFisher Scientific).
Statistical analysis
Each experiment was performed with at least 3 independent replicates and results were presented as mean ± SEM. Statistical analysis of data and presentation of results was performed using GraphPad Prism 9.0. The data between groups were compared by one-way analysis of variance (ANOVA) or a two-tailed unpaired Student's t-test, and the difference was statistically significant at P < 0.05.
Results
The expression levels of APC and PLA2R1 in clinical and podocyte models of membranous nephropathy
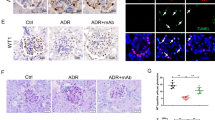
In order to detect the expression of APC and PLA2R1 in patients with IMN, we randomly selected 30 IMN patients and healthy volunteers. We measured the APC and PLA2R1 expression in the serum of IMN patients by qPCR and found that the expression level of APC decreased while the expression level of PLA2R1 increased (Fig. 1A). The protein level of PLA2R1 in serum was detected using ELISA and the results showed that it was also significantly elevated (Fig. 1B). The urine protein levels of IMN patients were tested to be markedly raised (Fig. 1C). To characterize the pathomorphology of patients with membranous nephropathy, we performed histomorphological observation, Masson staining, HE, PASM, PAS immunofluorescence and electron microscopic analysis of the patients' tissues, respectively. The findings revealed vacuolar degeneration of the renal tubular epithelium and inflammatory cell infiltration in the renal interstitium in patients with IMN (Fig. 1D). APC has a protective effect on cell survival, and PLA2R1 has been identified as the main target antigen in patients with IMN. To further study the interaction between APC and PLA2R1 in IMN, we established an IMN model in vitro by adding 100 μL/mL of enzymatically activated serum (ZAS) to the culture medium of human renal podocytes to treat 0, 4, 8, 12 and 24 h. Flow cytometry was used to detect the apoptosis of cells after ZAS treatment, and the results showed that more than 40% of the cells were apoptotic after 24 h of ZAS treatment, the results indicated that ZAS treatment resulted in apoptosis of human renal podocytes (Fig. 1E). In addition, TUNEL assay also indicated that ZAS treatment led to increased apoptosis of human renal podocytes (Fig. 1F). Consistent with our detection of a significant increase in the level of Caspase-3 in human renal podocytes after ZAS treatment (Fig. 1G). Next, we detected the expression levels of APC and PLA2R1 by Western blotting (WB) in the established IMN podocyte model in vitro, and found that APC protein level decreased and PLA2R1 protein level increased after ZAS treatment (Fig. 1H). This indicates that we have successfully established an in vitro model of IMN.
Detection of the expression of APC and PLA2R1 in the clinical level and cell model of IMN. A The expression levels of APC and PLA2R1 was detected by qPCR in clinical patients with IMN, n = 30 in each group. B The protein level of PLA2R1 in serum was detected using ELISA, n = 30 in each group. C The urine protein levels of IMN patients were tested using a non-interfering protein assay kit, n = 30 in each group. D Histomorphological analysis of IMN patients. E Cell apoptosis was detected by flow cytometry and the percentage of apoptosis was calculated, n = 3 in each group. F TUNEL assay for detecting apoptosis in ZAS-induced IMN podocyte model. G Caspase-3 activity kit was used to measure Caspase-3 activity in ZAS-induced IMN podocyte model, n = 3 in each group. H Western blot assay was used to detect the protein expression levels of APC and PLA2R1, n = 3 in each group. The measurement data were presented as Mean ± SD was applied to express data. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 μm for Masson, HE, PAS staining, and 50 μm for PASM staining. 0.5 μm and 0.2 μm for electron microscope
APC treatment inhibits podocyte apoptosis in ZAS-induced IMN model through increased ERK1/2 phosphorylation
What is the correlation between the low expression APC and apoptosis in IMN patients. Whether increasing the level of APC will inhibit the apoptosis of podocytes. To answer these questions, we added different concentrations of APC to the in vitro IMN model (100 μL/mL ZAS treated human renal podocytes for 24 h) to observe the effect on podocyte apoptosis. The control group was normal human renal podocytes without ZAS induction. We found that the apoptosis rate of podocytes decreased gradually with the increase of APC concentration (Fig. 2A). Besides, TUNEL assay also indicated that the increase of APC concentration treatment led to decreased apoptosis of IMN model (Fig. 2B). Likewise, the activity level of Caspase-3 was significantly reduced after APC treatment (Fig. 2C). These results suggest that APC can inhibit apoptosis in an in vitro IMN cell model. Previous studies have found that APC can activate the phosphorylation activity of ERK1/2 to regulate a series of biological functions in tumors, acute pancreatitis and vascular endothelial cells [17, 25, 26]. We detected ERK1/2 phosphorylation levels with specific ERK1/2 phosphorylation antibody and found that the phosphorylation levels of ERK1/2 were significantly increased in podocytes with increasing concentrations of APC (Fig. 2D). Thus, we reported that increasing the concentration of APC can activate the activity of ERK1/2 to inhibit ZAS-induced apoptosis of podocytes in vitro.
APC inhibits ZAS-induced podocyte apoptosis through ERK1/2 pathway. A Cell apoptosis was detected by flow cytometry after different concentrations of APC and the percentage of apoptosis was calculated. B TUNEL assay was performed to detect apoptosis in the APC-treated IMN podocyte model. C The Caspase-3 activity kit was used to measure the Caspase-3 activity in the IMN podocyte model after different APC treatment concentrations. D Western blot assay was used to detect the expression levels of ERK1/2 and p-ERK1/2 in the IMN podocyte model after different APC concentrations. The measurement data were presented as Mean ± SD was applied to express data. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 μm for TUNEL
ERK1/2 ameliorates IMN by promoting YB-1 entry into the nucleus in a podocyte model
To further study the direct relationship between the activity of ERK1/2 and the apoptosis of IMN in vitro cell model, we treated the cells with ERK1/2 inhibitor SCH772984. We found that the inhibitory effect of APC on apoptosis of podocytes was reversed by SCH772984 after adding APC and SCH772984 at the same time (Fig. 3A). Moreover, TUNEL assay also displayed increased apoptosis in the IMN model after the addition of SCH772984 (Fig. 3B). The activity level of Caspase-3 was significantly increased after the simultaneous treatment of APC and SCH772984 (Fig. 3C). These indicate that APC activates the activity of ERK1/2 to suppress apoptosis in IMN, and the inhibition of ERK1/2 activity has an impact on the blocking effect of APC on apoptosis. Recent study has shown that APC can promote YB-1 translocating into the nucleus through ERK1/2 activation [ Thus, our study is the first to report that APC can ameliorate membranous nephropathy by affecting podocyte apoptosis through the ERK1/2/YB-1/PLA2R1 axis. Idiopathic membranous nephropathy Activated protein C Zymosan activated serum Extracellular signal-regulated kinases 1/2 Y-box-binding protein 1 M-type receptor for secreted phospholipase A2 Neuroepidermal growth factor-like 1 protein Thrombospondin type 1 domain-containing 7A Phosphoinositide 3-Kinase RAC (Rho family)-alpha serine/threonine-protein kinase Mammalian target of rapamycin Endothelial protein C rece Proteinase-activated receptor 1 Myocyte enhancer-binding factor 2B OTU deubiquitinase, ubiquitin aldehyde binding 1 Yang L, Chen X, Li C, Xu P, Mao W, Liang X, Zuo Q, Ma W, Guo X, Bao K (2022) Real-world effects of Chinese herbal medicine for idiopathic membranous nephropathy (REACH-MN): protocol of a registry-based cohort study. Front Pharmacol 12:760482 Couser WG (2017) Primary membranous nephropathy. Clin J Am Soc Nephrol 12:983–997 Alsharhan L, Beck LH (2021) Membranous nephropathy: core curriculum 2021. Am J Kidney Dis 77:440–453 Wu L, Lai J, Ling Y, Weng Y, Zhou S, Wu S, Jiang S, Ding X, ** X, Yu K (2021) A review of the current practice of diagnosis and treatment of idiopathic membranous nephropathy in China. Med Sci Monit 27:e930097–e930101 Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361:11–21 Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay A-S (2014) Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371:2277–2287 Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, Ravindran A, Buob D, Jadoul M, Fervenza FC (2020) Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97:163–174 Liu W, Gao C, Dai H, Dong Z, Zheng Y, Gao Y, Liu Z, Feng Z, Tian X, Liu B (2020) Idiopathic membranous nephropathy: glomerular pathological pattern caused by extrarenal immunity activity. Front Immunol 11:2560–2572 Liu W, Gao C, Dai H, Zheng Y, Dong Z, Gao Y, Liu F, Zhang Z, Liu Z, Liu W, Liu B, Liu Q, Shi J (2019) Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front Immunol 10:1809 Lambeau G, Lazdunski M (1999) Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol Sci 20:162–170 Dong Y, Cao L, Tang H, Shi X, He Y (2017) Structure of human M-type phospholipase A2 receptor revealed by cryo-electron microscopy. J Mol Biol 429:3825–3835 Kerjaschki D (1992) Molecular pathogenesis of membranous nephropathy. Kidney Int 41:1090–1105 Liu D, Liu F, Wang X, Qiao Y, Pan S, Yang Y, Hu Y, Zhang Y, Tian F, Liu Z (2018) MiR-130a-5p prevents angiotensin II-induced podocyte apoptosis by modulating M-type phospholipase A2 receptor. Cell Cycle 17:2484–2495 Chiou TT-Y, Chau Y-Y, Chen J-B, Hsu H-H, Hung S-P, Lee W-C (2021) Rapamycin attenuates PLA2R activation-mediated podocyte apoptosis via the PI3K/AKT/mTOR pathway. Biomed Pharmacother 144:112349 Griffin JH, Zlokovic BV, Mosnier LO (2018) Activated protein C, protease activated receptor 1, and neuroprotection. Blood 132:159–169 Griffin JH, Fernández JA, Gale AJ, Mosnier LO (2007) Activated protein C. J Thromb Haemost 5:73–80 Guitton C, Cottereau A, Gérard N, Quillard T, Chauveau A, Devallière J, Tonnerre P, Charreau B (2011) Protective cross talk between activated protein C and TNF signaling in vascular endothelial cells: implication of EPCR, noncanonical NF-κB, and ERK1/2 MAP kinases. Am J Physiol Cell Physiol 300:C833–C842 Zhong X, Wang T, **e Y, Wang M, Zhang W, Dai L, Lai J, Nie X, He X, Madhusudhan T (2021) Activated protein C ameliorates diabetic cardiomyopathy via modulating OTUB1/YB-1/MEF2B axis. Front Cardiovasc Med 8:2000562 Bock F, Shahzad K, Wang H, Stoyanov S, Wolter J, Dong W, Pelicci PG, Kashif M, Ranjan S, Schmidt S (2013) Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci USA 110:648–653 Lyabin DN, Eliseeva IA, Ovchinnikov LP (2014) YB-1 protein: functions and regulation. WIREs RNA 5:95–110 Shah A, Lindquist JA, Rosendahl L, Schmitz I, Mertens PR (2021) Novel insights into YB-1 signaling and cell death decisions. Cancers 13:3306 Gibbert L, Hermert D, Wang J, Breitkopf D, Alidousty C, Neusser M, Cohen CD, Gröne E, Macheleidt I, Rauen T, Braun GS, Floege J, Ostendorf T, Raffetseder U (2018) YB-1 increases glomerular, but decreases interstitial fibrosis in CNI-induced nephropathy. Clin Immunol 194:67–74 Ishikawa S, Tsukada H, Bhattacharya J (1993) Soluble complex of complement increases hydraulic conductivity in single microvessels of rat lung. J Clin Invest 91:103–109 Liu WJ, Li ZH, Chen XC, Zhao XL, Zhong Z, Yang C, Wu HL, An N, Li WY, Liu HF (2017) Blockage of the lysosome-dependent autophagic pathway contributes to complement membrane attack complex-induced podocyte injury in idiopathic membranous nephropathy. Sci Rep 7:8643 Chen P, Zhang Y, Qiao M, Yuan Y (2007) Activated protein C, an anticoagulant polypeptide, ameliorates severe acute pancreatitis via regulation of mitogen-activated protein kinases. J Gastroenterol 42:887–896 O’Brien LA, Richardson MA, Mehrbod SF, Berg DT, Gerlitz B, Gupta A, Grinnell BW (2007) Activated protein C decreases tumor necrosis factor–related apoptosis-inducing ligand by an EPCR-independent mechanism involving Egr-1/Erk-1/2 activation. Arterioscler Thromb Vasc Biol 27:2634–2641 Zhong X, Wang T, Zhang W, Wang M, **e Y, Dai L, He X, Madhusudhan T, Zeng H, Wang H (2022) ERK/RSK-mediated phosphorylation of Y-box binding protein-1 aggravates diabetic cardiomyopathy by suppressing its interaction with deubiquitinase OTUB1. J Biol Chem 298:101989 Tomas NM, Beck LH, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay A-S, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G (2014) Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371:2277–2287 Zhang P, Huang W, Zheng Q, Tang J, Dong Z, Jiang Y, Liu Y, Liu W (2021) A novel insight into the role of PLA2R and THSD7A in membranous nephropathy. J Immunol Res 2021:8163298 Hoxha E, Reinhard L, Stahl RAK (2022) Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol 18:466–478 Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147 Rybalkina EY, Moiseeva NI (2022) Role of YB-1 Protein in Inflammation. Biochemistry 87:S94–S102 We would like to give our sincere gratitude to the reviewers for their constructive comments. This work was supported by National Natural Science Foundation of China (81860133), Natural Science Foundation of Jiangxi Provincial Department of Science and Technology (20192BAB205022) and the central government guides local science and technology development projects (20192ZDG04041). BK: Conceptualization, Methodology, Writing—Original draft preparation, Investigation, Validation, Visualization, Supervision, Writing—Reviewing and Editing. WS: Data curation. YL: Software. JH: Data curation. WT: Software. XF: Conceptualization, Writing—Original draft preparation, Supervision, Writing—Reviewing and Editing. The authors declare no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Ke, B., Shen, W., Liao, Y. et al. APC ameliorates idiopathic membranous nephropathy by affecting podocyte apoptosis through the ERK1/2/YB-1/PLA2R1 axis.
Mol Cell Biochem 478, 1999–2011 (2023). https://doi.org/10.1007/s11010-022-04650-7 Received: Accepted: Published: Issue Date: DOI: https://doi.org/10.1007/s11010-022-04650-7Data availability
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Competing interests
Additional information
Publisher's Note
Rights and permissions
About this article
Cite this article
Keywords