Abstract
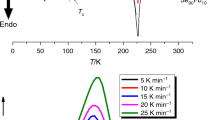
Kinetic studies and thermal stability become a crucial characteristic for switching in high-temperature regions in nanoscale phase-change materials, which are used as applications for non-volatile next-generation storage class memory. Differential scanning calorimetry has been used for theoretical and experimental studies of the glass transition kinetics, crystallization kinetics and thermal stability of newly synthesized Te(1−x) (GeSe0.5) Scx (x = 0.05, 0.1, 0.15) glasses. Sc has been used as a chemical modifier. The impact of rising Sc amount has been explained by relating the structural relaxation kinematics during glass transition process and devitrification during crystallization process in chalcogenide glasses and their various physicochemical properties. There is observable increase in crystallization rate by Sc incorporation. Te(1−x) (GeSe0.5) Scx material fragility index reveals that the composition is consistent with a potent glass-forming liquid. Heterogeneous nucleation occurs for the composition under study and is then followed by a two- or three-dimensional crystal development phenomena by means of the mean values of kinetic exponent factors. By adding Sc, it has been observed that the average values of the heat of atomization and mean bond energy decrease as the cohesive energy of the samples decreases. Inverse relation has been noticed between thermal stability parameter and enthalpy released while transformation of glass and crystalline phases.















Similar content being viewed by others
References
Redaelli A. Phase change memory: device physics, reliability and applications. Berlin: Springer; 2017.
Agarwal S, Lohia P, Dwivedi DK. Emerging phase change memory devices using non-oxide semiconducting glasses. J Non Cryst Solids. 2022;597:121874.
Le Gallo M, Sebastian A. An overview of phase-change memory device physics. J Phys D Appl Phys. 2020;53:213002.
Rostron P, Gaber S, Gaber D. Raman spectroscopy, review. Laser. 2016;21:24.
Tawfiq ZH, Fakhri MA, Adnan SA. Photonic crystal fibres PCF for different sensors in review. In: IOP Conf Ser Mater Sci Eng. 2018;454.
Singh PK, Dwivedi DK. Chalcogenide glass: fabrication techniques, properties and applications. Ferroelectrics. 2017;520:256–73.
Zhang W, Mazzarello R, Wuttig M, Ma E. Designing crystallization in phase-change materials for universal memory and neuro-inspired computing. Nat Rev Mater. 2019;4:150–68. https://doi.org/10.1038/s41578-018-0076-x.
Agarwal S, Lohia P, Dwivedi DK. Theoretical and experimental studies of crystallization and glass transition kinetics of GeTeSe chalcogenide glass for PCM. Phys B Condens Matter. 2022;646:414329.
Durai S, Raj S, Manivannan A. Impact of thermal boundary resistance on the performance and scaling of phase-change memory device. IEEE Trans Comput Aided Des Integr Circuits Syst. 2020;39:1834–40.
Sun X, Yu B, Ng G, Meyyappan M. One-dimensional phase-change nanostructure: germanium telluride nanowire. J Phys Chem C. 2007;111:2421–5.
Singh PK, Dwivedi DK. Influence of composition on structural properties and optical parameters of thermally evaporated Ge10-xSe60Te30Inx (0 ≤ x ≤ 6) thin films. Ferroelectrics. 2018;531:72–83. https://doi.org/10.1080/00150193.2018.1497418.
Peng L, Li Z, Wang G, Zhou J, Mazzarello R, Sun Z. Reduction in thermal conductivity of Sb2Te phase-change material by scandium/yttrium do**. J Alloys Compd. 2020;821:153499.
Park S, Park D, Jeong K, Kim T, Park S, Ahn M, et al. Effect of the thermal conductivity on resistive switching in GeTe and Ge2Sb2Te5 nanowires. ACS Appl Mater Interfaces. 2015;7:21819–27.
Dwivedi DK, Pathak HP, Kumar V, Shukla N. Effect of thermal annealing on structure and optical band gap of amorphous Se72Te25Sb3 thin films. In: AIP conf proc. American Institute of Physics Inc.; 2014. p. 719–21.
Wang Y, Wang T, Liu G, Guo T, Li T, Lv S, et al. High thermal stability and fast speed phase change memory by optimizing GeSbTe with Scandium do**. Scr Mater. 2019;164:25–9.
Wang Y, Zheng Y, Liu G, Li T, Guo T, Cheng Y, et al. Scandium doped Ge2Sb2Te5 for high-speed and low-power-consumption phase change memory. Appl Phys Lett. 2018;112:133104.
Chen X, Zheng Y, Zhu M, Ren K, Wang Y, Li T, et al. Scandium do** brings speed improvement in Sb2Te alloy for phase change random access memory application. Sci Rep. 2018;8:6839.
Kumar S, Sharma V. Structural transition on do** rare earth Sm to Ge2Sb2Te5 phase change material. J Alloys Compd. 2021;877:160246. https://doi.org/10.1016/j.jallcom.2021.160246.
Chen Y, Chen N, Chen B, Zhang Q, Li X, Deng Q, et al. Electrical properties and structural transition of Ge2Sb2Te5 adjusted by rare-earth element Gd for nonvolatile phase-change memory. J Appl Phys. 2018;124:145107.
Rao V, Singh PK, Lohia P, Dwivedi DK. Non-isothermal crystallization kinetics of Se82−xTe18Gex (0 ≤ x ≤ 12) for memory applications. Indian J Phys. 2022;96:1075–85.
Karpov VG, Kryukov YA, Mitra M, Karpov IV. Crystal nucleation in glasses of phase change memory. J Appl Phys. 2008;104:054507.
Liu F, Song SJ, Xu JF, Wang J. Determination of nucleation and growth modes from evaluation of transformed fraction in solid-state transformation. Acta Mater. 2008;56:6003–12. https://doi.org/10.1016/j.actamat.2008.08.011.
Sebastian A, Le Gallo M, Krebs D. Crystal growth within a phase change memory cell. Nat Commun. 2014;5:1–9. https://doi.org/10.1038/ncomms5314.
Vázquez J, Barreda DGG, López-Alemany PL, Villares P, Jiménez-Garay R. A study on non-isothermal transformation kinetics: application to the crystallization of the Ge0.18Sb0.23Se0.59 glassy alloy. Mater Chem Phys. 2006;96:107–15.
Lafi OA, Imran MMA, Abdullah MK. Glass transition activation energy, glass-forming ability and thermal stability of Se90In10-xSnx (x = 2, 4, 6 and 8) chalcogenide glasses. Phys B Condens Matter. 2007;395:69–75.
Lankhorst MHR. Modelling glass transition temperatures of chalcogenide glasses. Applied to phase-change optical recording materials. J Non Cryst Solids. 2002;297:210–9.
Cárdenas-Leal JL, Vázquez J, Barreda DGG, González-Palma R, López-Alemany PL, Villares P. Analysis of the glass-crystal transformation kinetics by means of the theoretical method developed (TMD) under both non-isothermal and isothermal regimes. Application to the crystallization of the Ag0.16As0.34Se0.50 glassy alloy. J Alloys Compd. 2015;622:610–7.
Moynihan CT, Easteal AJ, Wilder J, Tucker J. Dependence of the glass transition temperature on heating and cooling rate. J Phys Chem. 1974;78:2673–7.
Ruitenberg G. Applying Kissinger analysis to the glass transition peak in amorphous metals. Thermochim Acta. 2003;404:207–11.
Srivastava A, Chandel N, Mehta N. Iso-conversional kinetic analysis of quaternary glass re-crystallization. Heliyon. 2017;3:e00249. https://doi.org/10.1016/j.heliyon.2017.e00249.
Lad KN, Savalia RT, Pratap A, Dey GK, Banerjee S. Isokinetic and isoconversional study of crystallization kinetics of a Zr-based metallic glass. Thermochim Acta. 2008;473:74–80.
Abdel-Rahim MA, Hafiz MM, Mahmoud AZ. Crystallization kinetics of overlap** phases in Se70Te15Sb15 using isoconversional methods. Prog Nat Sci Mater Int. 2015;25:169–77.
Cárdenas-Leal JL, Vázquez J, López-Alemany PL, Villares P, Jiménez-Garay R. A study on the non-isothermal transformation kinetics of glassy alloys when the nucleation frequency and crystal growth rate depend on time as a power law. Application to the crystallization of the Ag0.16As0.42Se0.42 semiconductor glass. J Alloys Compd. 2009;471:44–51.
Henderson DW. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non Cryst Solids. 1979;30:301–15.
Yan Z, Dang S, Wang X, Lian P. Applicability of Johnson-Mehl-Avrami model to crystallization kinetics of Zr60Al15Ni25 bulk amorphous alloy. Trans Nonferrous Metals Soc China (Engl Ed). 2008;18:138–44.
Joraid AA. Limitation of the Johnson–Mehl–Avrami (JMA) formula for kinetic analysis of the crystallization of a chalcogenide glass. Thermochim Acta. 2005;436:78–82.
Schick C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal Bioanal Chem. 2009;395:1589–611.
Sharma A, Kumar H, Mehta N. Determination of specific heat in multi-component chalcogenide glasses of SeTeSnPb system using modulated differential scanning calorimetry. Mater Lett. 2012;86:54–7.
Lasocka M. The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. Mater Sci Eng. 1976;23:173–7.
Kauzmann W. The nature of the glassy state and the behavior of liquids at low temperatures.
Ichitsubo T, Matsubara E, Miyagi K, Itaka W, Tanaka K, Hosokawa S. Low-temperature elastic moduli of a Pd-based metallic glass showing positive phonon dispersion. Phys Rev B Condens Matter Mater Phys. 2008;78:052202.
Selvanathan D, Bresser WJ, Boolchand P. Stiffness transitions in SixSe1-x glasses from Raman scattering and temperature-modulated differential scanning calorimetry.
Boolchand P, Georgiev DG, Goodman B. Discovery of the intermediate phase in chalcogenide glasses. J Optoelectron Adv Mater. 2001;3:703–20.
Georgiev DG, Boolchand P, Micoulaut M. Rigidity transitions and molecular structure of AsxSe1-x glasses. 2000.
Tanwar N, Saraswat YK, Saraswat VK. Enthalpy and entropy change during glass/crystal phase transformation for GeySe94-yIn6 (y = 10, 15 and 20) glasses. J Ovonic Res. 2015;11:183–8.
Saraswat V, Pal SK, Mehta N, Kumar A, Imran MMA. Thermal analysis of novel third-generation phase-change materials with zinc as a chemical modifier. RSC Adv. 2023;13:3602–11.
Rao V, Dwivedi DK. Glass transition kinetics and thermal stability of Se82-xTe18Sbx (x = 0, 4, 8 and 12 at %) glassy alloys. J Mater Sci Mater Electron. 2017;28:6208–16.
Vyazovkin S, Linert W. Kinetic analysis of reversible thermal decomposition of solids. Int J Chem Kinet. 1995;27:73–84.
Khawam A, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Nonisothermal kinetic studies. Thermochim Acta. 2005;436:101–12.
Kissinger HE. Reaction kinetics in differential thermal analysis. Helv Chim Acta. 1956;29:1702–6.
Augis JA, Bennett JE. calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Matusita K, Sakka S. Kinetic study on non-isothermal crystallization of glass by thermal analysis. Bull Inst Chem Res Kyoto Univ. 1981;59(3):159–71.
Kumar H, Mehta N, Singh K. Calorimetric studies of glass transition phenomenon in glassy Se80-xTe20Snx alloys. Phys Scr. 2009;80: 065602.
Toluchuri SR. On the fragility of Al70Ni10Ti10Zr5Ta5 alloy powder. 2019.
Tanaka K. Structural phase transitions in chalcogenide glasses. Phys Rev B. 1989;39:1270–9.
Abd-el Salam MN, Shaaban ER, Benabdallah F, Hussein AMA, Mohamed M. Experimental and theoretical studies of glass and crystallization kinetics of semiconducting As40Se40Ag20 chalcogenide glass. Phys B Condens Matter. 2021;608: 412745.
Chandel N, Mehta N. Analysis of physicochemical properties in covalent network chalcogenide glasses (ChGs): critical review of theoretical modeling of chemical bond approach. SN Appl Sci. 2019;1:1–14.
Shaaban ER, Kansal I, Shapaan M, Ferreira JMF. Thermal stability and crystallization kinetics of ternary Se–Te–Sb semiconducting glassy alloys. J Therm Anal Calorim. 2009;98:347–54.
Tich L, Tichfi H. Covalent bond approach to the glass-transition temperature of chalcogenide glasses. J Non Cryst Solids. 1995;189:141–6.
Liu J, Wang E, Zhao Y, Xu X, Moon JS, Anantram MP. Impact of do** on bonding energy hierarchy and melting of phase change materials. J Appl Phys. 2018;124:094503.
Gutiérrez-Oliva S, Jaque P, Toro-Labbé A. Using Sanderson’s principle to estimate global electronic properties and bond energies of hydrogen-bonded complexes. J Phys Chem A. 2000;104:8955–64.
Qiao C, Xu M, Wang S, Wang CZ, Ho KM, Miao X, et al. Structure, bonding nature and transition dynamics of amorphous Te. Scr Mater. 2021;202:114011. https://doi.org/10.1016/j.scriptamat.2021.114011.
Xu M, Cheng YQ, Sheng HW, Ma E. Nature of atomic bonding and atomic structure in the phase-change Ge2Sb2Te5 Glass. Phys Rev Lett. 2009;103:2–5.
Saad M, Poulain M. Glass forming ability criterion. Mater Sci Forum. 1987;19–20:11–8.
Acknowledgements
The authors are appreciative of the financial support provided by CST-UP through major research project ID-559 reference number CST-UP D/2286. We are grateful to the several scientists and researchers for their insightful publications and research we used to prepare the current manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agarwal, S., Lohia, P. & Dwivedi, D.K. Kinetics study and thermal analysis of novel phase-change materials with scandium as chemical modifier. J Therm Anal Calorim 148, 10777–10793 (2023). https://doi.org/10.1007/s10973-023-12440-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12440-6