Abstract
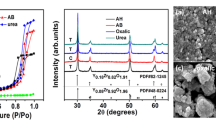
The nanocrystalline Zirconolite (CaZrTi2O7 or CZT) powder was successfully synthesized at low temperature using wet chemical method. The calcination temperature was estimated by simultaneous thermogravimetry and differential thermal analysis (TG–DTA). The calcination of the precursor at the various temperatures (600–1250 °C) exhibited two major phases of nanocrystalline CZT powder. The XRD pattern has confirmed the formation of the fluorite structure at 600–900 °C. However, the fluorite structure of the CZT was completely transformed to monoclinic (2 M) structure at 1000 °C. Further, SEM, and TEM have investigated the influence of annealing temperatures on the microstructures and particle size. The linear thermal expansion of sintered CZT pellet was measured by dilatometrically in the range of 30–950 °C and the calculated thermal expansion coefficient value was (α) = 9.93 × 10–6 K−1. The theoretical density > 95% was achieved in nanocrystalline CZT powder after being pelletized and sintered at 1250 °C for 5 h. The thermal expansion coefficient and density of the CZT are favorable for potential application in the immobilization of radwaste containing actinide and lanthanides.






Similar content being viewed by others
References
Ringwood AE, Kesson SE, Ware NG, Hibberson W, Major A. Immobilization of high-level nuclear reactor wastes in SYNROC. Nature. 1979;278:219–23.
Ringwood AE, Oversby VM, Kesson SE, Sinclair W, Ware N, Hibberson W. Immobilization of high-level nuclear reactor wastes in SYNROC: a current appraisal. Nucl Chem Waste Manag. 1981;2(4):287–305.
Gatehouse BM, Grey IE, Hill RJ, Rossell HJ. Zirconolite, CaZrxTi3-xO7; structure refinements for near-end-member compositions with x = 0.85 and 1.30. Acta Cryst. 1981;B37:306–12.
Cheary RW, Coelho AA. A site occupancy analysis of zirconolite CaZrxTi3–xO7. Phys Chem Minerals. 1997;24(6):447–54.
Cheary RW. Zirconolite CaZr0.92Ti2.08O7 from 294 to 1173 K. J Solid State Chem. 1992;98(2):323–9.
Rossell HJ. Zirconolite-a fluorite-related superstructure. Nature. 1980;283(5744):282–3.
White TJ, Segall RL, Hutchison JL, Barry JC. Polytypic behavior of zirconolite. Proc R Soc Lond A. 1984;392:343–58.
Lumpkin GR. Ceramic waste forms for actinides. Elements. 2006;2(6):365–72.
Wang L, Liang T. Ceramics for high level radioactive waste solidification. J Adv Ceram. 2012;1(3):194–203.
Whittle KR, Hyatt NC, Smith KL, Margiolaki I, Berry FJ, Knight KS, Lumpkin GR. Combined neutron and X-ray diffraction determination of disorder in doped zirconolite-2M. Am Mineral. 2012;97:291–8.
Matzke HJ, Ray ILF, Seatonberry BW, Thiele H, Trisoglio C, Walker CT, White TJ. Incorporation of transuranic elements in titanate nuclear waste ceramics. J Am Ceram Soc. 1990;73:370–8.
Meng C, Ding X, Li W, Zhao J, Yang H. Phase structure evolution and chemical durability studies of Ce-doped zirconolite–pyrochlore synroc for radioactive waste storage. J Mate Sci. 2016;51(11):5207–15.
Vance ER, Lumpkin GR, Carter ML, Cassidy DJ, Ball CJ, Day RA, Begg BD. Incorporation of uranium in zirconolite (CaZrTi2O7). J Am Ceram Soc. 2004;85(7):1853–9.
Blackburn LR, Bailey DJ, Sun S, Gardner LJ, Stennett MC, Corkhill CL. Hyatt NC Review of zirconolite crystal chemistry and aqueous durability. Advs Appl Ceram. 2021;120(2):69–83.
Ringwood AE, Kesson SE, Ware NG, Hibberson WO, Major A. The SYNROC process: a geochemical approach to nuclear waste immobilization. Geochem J. 1979;13(4):141–65.
Gieré R, Malmström J, Reusser E, Lumpkin GR, Düggelin M, Mathys D, Guggenheim R, Günther D. Durability of zirconolite in hydrothermal fluids: implications for nuclear waste disposal. Mater Res Soc Symp Proc. 2000;663:267.
Zhang Y, Stewart MWA, Li H, Carter ML, Vance ER, Moricca S. Zirconolite-rich titanate ceramics for immobilization of actinides—waste form/HIP can interactions and chemical durability. J Nucl Mater. 2009;395:69–74.
Lumpkin GR, Ewing RC, Chakoumakos BC, Greegor RB, Lytle FW, Foltyn EM, Clinard FW, Boatner LA, Abraham MM. Alpha-recoil damage in zirconolite (CaZrTi2O7). J Mater Res. 1986;1(4):564–76.
Vance ER, Ball CJ, Smith RA, Day KL, Blackford MG, Begg BD, Angel PJ. Actinide and rare earth incorporation into zirconolite. J Alloys Compds. 1994;213–214:406–9.
Begg BD, Vance ER, Day RA, Hambley M, Conradson SD. Plutonium and neptunium incorporation in zirconolite. Mater Res Soc Symp Proc. 1996;465–325.
Begg BD, Vance ER, Lumpkin GR. Charge compensation and the incorporation of cerium in Zirconolite and Perovskite. MRS Online Proc Library. 1997;506:79–86.
Begg BD, Vance ER, Conradson SD. The incorporation of plutonium and neptunium in Zirconolite and Perovskite. J Alloys Compds. 1998;271–273:221–6.
Omel’yanenko BI, Livshits TS, Yudintsev SV, Nikonov BS. Natural and artificial minerals as matrices for immobilization of actinides. Geology Ore Deposits. 2007;49(3):173–93.
Ventura GD, Bellatreccia F, Williams CT. Zirconolite with significant REEZrNb(Mn, Fe)O7 from a xenolith of the Laacher Eruptive Center, Eifel volcanic Region, Germany. Can Mineral. 2000;38(1):57–65.
Jorion F, Deschanels X, Advocat T, Desmouliere F, Cachia JN, Peuget S, Roudil D, Leturcq G. Zirconolite for minor actinide containment and alpha irradiation resistance. Nucl Sci Eng. 2006;153:262–71.
Bailey DJ, Lawson SM, Sun SK, Stennett MC, Lee TH, Ravel B, Corkhill CL, Heo J, Hyatt NC. A new approach to the immobilisation of technetium and transuranics: co-disposal in a zirconolite ceramic matrix. J Nucl Mater. 2020;528: 151885.
Wei ZJ, Bao WC, Sun SK, Blackburn LR, Tan SH, Gardner LJ, Guo WM, Xu F, Hyatt NC, Lin HT. Synthesis of zirconolite-2M ceramics for immobilisation of neptunium. Ceram Int. 2021;47(1):1047–52.
Fielding PE, White TJ. Crystal chemical incorporation of high-level waste species in aluminotitanate-based ceramics: valence, location, radiation damage, and hydrothermal durability. J Mater Res. 1987;2(3):387–414.
Yugo M, Kenji S, Akihiko K. Nanocrystalline CaZrTi2O7 Photo catalyst prepared by a polymerizable complex method in the presence of Cs2CO3 flux for water splitting. Chem Lett. 2009;38(2):180–1.
Chen Shifu LW, Mingsong J, Yunguang Y. Preparation, characterization, and activity evaluation of CaZrTi2O7 photo catalyst. Mater Chem Phys. 2012;134(2–3):951–7.
Sandeep K, Thomas JK, Solomon S. Synthesis and characterization of AZrTi2O7 (A = mg, ca, Sr and Ba) functional nanoceramics. J Electroceram. 2019;43:1–9.
Pöml P, Geisler T, Konings R. High-temperature heat capacity of zirconolite (CaZrTi2O7). J Chem Thermodyn. 2006;38(8):1013.
Subramani T, Baker J, Xu H, Navrotsky A. Synthesis, characterization, and enthalpies of formation of uranium substituted zirconolites. ACS Earth Space Chem. 2020;4(10):1878–87.
Salamat A, McMillan PF, Firth S, Woodhead K, Hector AL, Garbarino G, Stennett MC, Hyatt NC. Structural transformations and disordering in zirconolite (CaZrTi2O7) at high pressure. Inorg Chem. 2013;52(3):1550–8.
Jafar M, Achary SN, Salke NP, Sahu AK, Rao R, Tyagi AK. X-ray diffraction and Raman spectroscopic investigations on CaZrTi2O7-Y2Ti2O7 system: Delineation of phase fields consisting of potential ceramic host materials. J Nucl Mater. 2016;475:192–9.
Jafar M, Phapale S, Achary SN, Mishra R, Tyagi AK. High-temperature crystallographic and thermodynamic investigations on synthetic zirconolite (CaZrTi2O7). J Therm Anal. 2017;131:2709–18.
Vance ER, Ball CJ, Blackford MG, Cassidy DJ, Smith KL. Crystallization of zirconolite from an alkoxide precursor. J Nucl Mater. 1990;175(1–2):58–66.
Muthuraman M, Dhas NA, Patil KC. Combustion synthesis of oxide materials for nuclear waste immobilization. Bull Mater Sci. 1994;17(6):977–87.
Gilbert MR. Molten salt synthesis of zirconolite polytypes. MRS Online Proc Lib (OPL) Symposium NW—Scientific Basis for Nuclear Waste Management XXXVII. 2014; 1665:325–330.
Zhang K, Wen G, Jia Z, Teng Y, Zhang H. Self-propagating high-temperature synthesis of zirconolite using CuO and MoO3 as the oxidants. Int J Appl Ceram tech. 2015;12 S 3:E111.
Wang S, Song Z, Teng Y, Wang L. Novel method to synthesis of single phase zirconolite ceramic. Adv Appl Ceram. 2015;114(3):188–90.
Sun SK, Stennett MC, Corkhill CL, Hyatt NC. Reactive spark plasma synthesis of CaZrTi2O7 zirconolite ceramics for plutonium disposition. J Nucl Mater. 2018;500:11–4.
McCaugherty S, Grosvenor AP. Low-temperature synthesis of CaZrTi2O7 zirconolite-type materials using ceramic, coprecipitation, and sol–gel methods. J Mater Chem C. 2019;7:177–87.
Sandeep K, Thomas JK, Solomon S. Synthesis, and characterization of AZrTi2O7 (A = Mg, Ca, Sr, and Ba) functional nanoceramics. J Electroceramics. 2019;43:1–9.
Gorski A, Krasnicka A. Influence of the cation on the formation of free hydrogen and formaldehyde in the thermal decomposition of formats. J Therm Anal. 1987;32:1345–54.
Gorski A, Krasnicka A. Origin of organic gaseous formed in the thermal decomposition of formates. J Therm Anal. 1987;321:243–1251.
Muthuraman M, Patil KC. Sintering, microstructural and dilatometric studies of combustion synthesized synroc phases. Mater Res Bull. 1996;31(1):1375–81.
Roberts RB, White GK, Buykx WJ, Cassidy DJ. Thermal expansion of synroc minerals. J Nucl Mater. 1987;148:353–5.
Ball CJ, Thorogood GJ, Vance ER. Thermal expansion coefficients of zirconolite (CaZrTi2O7) and perovskite (CaTiO3) from X-ray powder diffraction analysis. J Nucl Mater. 1992;190(1):298–301.
Muthuraman M, Patil KC, Senbagaraman S, Umarji AM. Sintering, microstructural and dilatometric studies of combustion synthesized synroc phases. Mater Res Bull. 1996;31(1):1375–81.
Buykx WJ. Specific heat, thermal diffusivity and thermal conductivity of synroc, perovskite, zirconolite and barium hollandite. J Nucl Mater. 1982;107:78–82.
Acknowledgements
The SAIF facilities, IIT-B Mumbai and NCNNUM, University of Mumbai are specially acknowledged for their characterization facilities such as SEM, TEM and TGA etc. Mr. Harishchandra Nishad from NCNNUM has been acknowledged for SAED pattern indexing.
Author information
Authors and Affiliations
Contributions
BMP performed the synthesis and characterization and wrote the manuscript. GCW, ACC and PSW corrected the manuscript. All authors discussed the results, helped shape the research as well as read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, B.M., Wadhawa, G.C., Chaskar, A.C. et al. Low-temperature synthesis and thermal expansion of nanocrystalline monoclinic zirconolite ceramics. J Therm Anal Calorim 148, 7591–7596 (2023). https://doi.org/10.1007/s10973-023-12269-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12269-z