Abstract
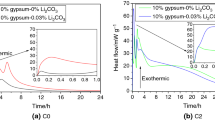
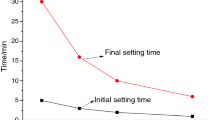
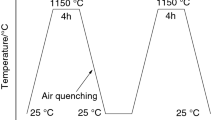
The circumstances in which calcium sulfoaluminate (CSA) cements used are multivariate. Considering the curing temperature greatly influence the formation of hydrates, the hydration of CSA cement containing increasing amount of anhydrite which cured at different temperature was analyzed by calorimetry, XRD and DTA-TGA. And the setting time and mechanical strength are measured to explore the correlation between the microstructure revolution and macroscopic performance. Results illustrate that the hydration of prepared CSA cement is highly dependent on the curing regimes and anhydrite dosage. The addition of anhydrite prolongs the setting of CSA cement at 5 °C, while it accelerates the setting at 20 °C and 40 °C. The usage of anhydrite is detrimental to strength development of CSA cement mortars which cured at 5 °C and 20 °C, but it’s the opposite at 40 °C. Additionally, elevated curing temperature favors the formation of ettringite, alumina gel and monosulfate within 1 d. CSA cement prepared with higher amount of anhydrite is beneficially used in the hot environment.








Similar content being viewed by others
References
Habert G. Environmental impact of Portland cement production. In: Pacheco-Torgal F, Jalali S, Labrincha J, John VM, editors. Eco Efficient Concrete. Woodhead Publishing; 2013; https://doi.org/10.1533/9780857098993.1.3.
Ioannou S, Reig L, Paine K, Quillin K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem Concr Res. 2014. https://doi.org/10.1016/j.cemconres.2013.09.015.
Berger S, Aouad G, Coumes CCD, Le Bescop P, Damidot D. Leaching of calcium sulfoaluminate cement pastes by water at regulated pH and temperature: Experimental investigation and modeling. Cem Concr Res. 2013. https://doi.org/10.1016/j.cemconres.2013.06.014.
Juenger MCG, Winnefeld F, Provis JL, Ideker JH. Advances in alternative cementitious binders. Cem Concr Res. 2011. https://doi.org/10.1016/j.cemconres.2010.11.012.
Shen Y, Qian J. Utilisation of phosphogypsum for sulfate-rich belite sulfoaluminate cement production. Adv Cem Res. 2015;27(9):515–25.
Mihelj NF, Ukrainczyk N, Leakovic S, Sipusic J. Waste phosphogypsum—toward sustainable reuse in calcium sulfoaluminate cement based building materials. Chem Biochem Eng Q. 2013;27(2):219–26.
Wu K, Shi H, Guo X. Utilization of municipal solid waste incineration fly ash for sulfoaluminate cement clinker production. Waste Manag. 2011;31(9):2001–8.
Wu K, Hu Y, Zhang L, Su Y, Han J, Yang Z, et al. Quantitative evaluation of interfacial transition zone of sustainable concrete with recycled and steel slag as aggregate. Struct Concr. 2020. https://doi.org/10.1002/suco.202000135.
Gartner E. Industrially interesting approaches to “low-CO2” cements. Cem Concr Res. 2004;34(9):1489–98.
Zhang L. Microstructure and performance of calcium sulfoaluminate cements. University of Aberdeen. 2000.https://doi.org/10.1155/2020/7564108.
Winnefeld F, Lothenbach B. Phase equilibria in the system Ca4Al6O12SO4–Ca2SiO4–CaSO4–H2O referring to the hydration of calcium sulfoaluminate cements. RILEM Tech Lett. 2016. https://doi.org/10.21809/rilemtechlett.2016.5.
Zhou Q, Glasser FP. Thermal stability and decomposition mechanisms of ettringite at < 120 °C. Cem Concr Res. 2001;31(9):1333–9.
Zhang L, Glasser FP. Hydration products and microstructure of calcium sulfoaluminate cement pastes in different curing regimes. In: Paper presented at the 19th international conference on cement chemistry, Bei**g, China, 2015.
Zhang L, Glasser F. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv Cem Res. 2002;14(4):141–56.
Berger S, Coumes CCD, Bescop PL, Damidot D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cem Concr Res. 2011;41:149–60.
Ambroise J, Pera J. Use of calcium sulfoaluminate cement to improve strength of mortars at low temperature. Concr Repair Rehabil Retrofit II. 2009;325–6:457.
Jen G, Stompinis N, Jones R. Chloride ingress in a belite-calcium sulfoaluminate cement matrix. Cem Concr Res. 2017. https://doi.org/10.1016/j.cemconres.2017.02.013.
Kaufmann J, Winnefeld F, Lothenbach B. Stability of ettringite in CSA cement at elevated temperatures. Adv Cem Res. 2015;28(4):251–561.
Xu L, Liu S, Li N, et al. Retardation effect of elevated temperature on the setting of calcium sulfoaluminate cement clinker. Constr Build Mater. 2018. https://doi.org/10.1016/j.conbuildmat.2018.05.061.
Winnefeld F, Lothenbach B. Hydration of calcium sulfoaluminate cements—experimental findings and thermodynamic modelling. Cem Concr Res. 2010. https://doi.org/10.1016/j.cemconres.2009.08.014.
Winnefeld F, Barlag S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J Therm Anal Calorim. 2010. https://doi.org/10.1007/s10973-009-0582-6.
Li H, Xue Z, Liang G, Wu K, Dong B, Wang W. Effect of C-S-Hs-PCE and sodium sulfate on the hydration kinetics and mechanical properties of cement paste. Constr Build Mater. 2021. https://doi.org/10.1016/j.conbuildmat.2020.121096.
Sun D, Shi H, Wu K, Miramini S, Li B, Zhang L. Influence of aggregate surface treatment on corrosion resistance of cement composite under chloride attack. Constr Build Mater. 2020. https://doi.org/10.1016/j.conbuildmat.2020.118636.
Xu L, Wu K, Li N, Zhou X, Wang P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J Clean Prod. 2017. https://doi.org/10.1016/j.jclepro.2017.05.055.
Winnefeld F, Barlag S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. Zkg Int. 2009;12:42–53.
Telesca A, Marroccoli M, Pace ML, Tomasulo M, Valenti GL, Monteiro PJM. A hydration study of various calcium sulfoaluminate cements. Cem Concr Compos. 2014. https://doi.org/10.1016/j.cemconcomp.2014.07.002.
Georgin JF, Prud’homme E. Hydration modelling of an ettringite-based binder. Cem Concr Res. 2015. https://doi.org/10.1016/j.cemconres.2015.05.009.
Perkins RB, Palmer CD. Solubility of ettringite (Ca6[Al(OH)6]2(SO4)3·26H2O) at 5–75 °C. Geochim Cosmochim Acta. 1999;63(13–14):1969–80.
Song F, Yu Z, Yang F, Lu Y, Liu Y. Microstructure of amorphous aluminum hydroxide in belite-calcium sulfoaluminate cement. Cem Concr Res. 2015;71:1–6.
Taylor HFW. Cement chemistry. In., 2 edn. Thomas Telford, London, 1997.
Chang J, Zhang Y, Shang X, Zhao J, Yu X. Effects of amorphous AH3 phase on mechanical properties and hydration process of \({\mathrm{C}}_{4}{\mathrm{A}}_{3}{\bar{\mathrm{S}}}{-}{\mathrm{C}{\bar{\mathrm{S}}}\mathrm{H}}_{2}{-}{\mathrm{CH}}{-}{\mathrm{H}_{2}}\mathrm{O}\) system. Constr Build Mater. 2017.https://doi.org/10.1016/j.conbuildmat.2016.11.111
Hu C, Hou D, Li Z. Micro-mechanical properties of calcium sulfoaluminate cement and the correlation with microstructures. Cem Concr Compos. 2017. https://doi.org/10.1016/j.cemconcomp.2017.02.005.
Wadsö L. Applications of an eight-channel isothermal conduction calorimeter for cement hydration studies. Cem Int. 2005;3(5):94–101.
Acknowledgements
The work was supported by the National Key Technology R&D Programs in the 13th Five-year Plan of China (2016YFC0700905), National Natural Science Fund of China (51878479, 51978505), Shanghai Rising Star Program (20QC1400600), Sichuan Science and Technology Program (2019YFSY0018) and Sichuan Huashi Group Co., LTD. Additionally, thanks are extended to the anonymous reviewers whose suggestions improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, L., Tang, C., Li, H. et al. Hydration characteristics assessment of a binary calcium sulfoaluminate-anhydrite cement related with environment temperature. J Therm Anal Calorim 147, 3053–3061 (2022). https://doi.org/10.1007/s10973-021-10730-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10730-5