Abstract
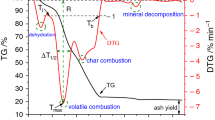
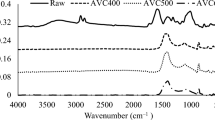
Antibiotic filter residue (AFR) is a typical deep-utilized biomass waste residue with high yield, which is difficult to burn be individually utilized as fuel due to its poor combustion characteristics. The co-combustion of AFR with vegetal biomass is likely to be an effective approach to enhance the combustion performance of AFR, while the co-firing mechanism and kinetics have yet to be fully understood. The present study aimed to investigate the co-combustion characteristics and kinetics of antibiotic filter residue and vegetal biomass (forestall biomass and agricultural biomass) by thermogravimetric experiments and kinetics analysis. The experimental results showed that the co-firing of AFR with peanut shell and poplar improved the combustion characteristics of AFR. Kinetic analysis indicated that the optimal solid-phase reaction models of the blend combustion were diffusion models. The specific form of the reaction function varied with the type and mass fraction of the blends. The present study can provide an improved understanding of the clean and efficient utilization of antibiotic filter residue by co-combustion.









Similar content being viewed by others
References
Su M, Chen C, Yang Z. Urban energy structure optimization at the sector scale: considering environmental impact based on life cycle assessment. J Clean Prod. 2016;112:1464–74.
Munawer ME. Human health and environmental impacts of coal combustion and post-combustion wastes. J Sustain Min. 2018;17(2):87–96.
Yao H, He B, Ding G, Tong W, Kuang Y. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal. Appl Therm Eng. 2019;156:708–21.
Niu YQ, Lv Y, Lei Y, Liu SQ, Liang Y, Wang DH, et al. Biomass torrefaction: properties, applications, challenges, and economy. Renew Sust Energy Rev. 2019;115:UNSP 109395.
Yao XW, Zhou HD, Xu KL, Xu QW, Li L. Investigation on the fusion characterization and melting kinetics of ashes from co-firing of anthracite and pine sawdust. Renew Energy. 2020;145:835–46.
Heidari M, Dutta A, Acharya B, Mahmud S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J Energy Inst. 2019;92(6):1779–99.
Smajevic I, Kazagic A. Development, significance and possibilities of application cofiring of coal with biomass in thermal power plant in BOSNIA and Herzegovina. In: Karabegovic I, editor. New technologies, development and application II. Lecture notes in networks and systems. Cham: Springer; 2020. p. 461–7.
Smith JS, Safferman SI, Saffron CM. Development and application of a decision support tool for biomass co-firing in existing coal-fired power plants. J Clean Prod. 2019;236:UNSP 117375.
Agbor E, Zhang X, Kumar A. A review of biomass co-firing in North America. Renew Sust Energy Rev. 2014;40:930–43.
Robinson AL, Junker H, Baxter LL. Pilot-scale investigation of the influence of coal-biomass cofiring on ash deposition. Energy Fuels. 2002;16(2):343–55.
Chowdhury MSR, Azad AK, Karim MR, Naser J, Bhuiyan AA. Reduction of GHG emissions by utilizing biomass co-firing in a swirl-stabilized furnace. Renew Energy. 2019;143:1201–9.
Bhuiyan AA, Blicblau AS, Islam AKMS, Naser J. A review on thermo-chemical characteristics of coal/biomass co-firing in industrial furnace. J Energy Inst. 2018;91(1):1–18.
**n SZ, Huang F, Liu XY, Mi T, Xu QL. Torrefaction of herbal medicine wastes: characterization of the physicochemical properties and combustion behaviors. Bioresour Technol. 2019;287:7.
Zhuang X, Zhan H, Huang Y, Song Y, Yin X, Wu C. Influence of hydrothermal upgrading on the fuel characteristics and combustion behavior of herb wastes. J Fuel Chem Technol. 2018;46(8):940–9.
**n S, Huang F, Liu X, Xu Q, Mi T. Pyrolysis and combustion characteristics and kinetics of torrefied traditional Chinese medicine waste. CIESC. 2019;70(8):3142–50.
Liu B, Jiang X, Lu G, Wang F, Chi Y, Yan J. Thermal dynamics model analysis of combustion and evolved gas analysis of dregs/coal disposal. J Combust Sci Technol. 2015;21(2):150–6.
Ma LY, Wang DM, **n HH, Qi XY, Dou GL. The competitive reaction mechanism between oxidation and pyrolysis consumption during low-rank coal combustion at lean-oxygen conditions: a quantitative calculation based on thermogravimetric analyses. Can J Chem Eng. 2018;96(12):2575–85.
Kok MV, Varfolomeev MA, Nurgaliev DK. Isoconversional methods to determine the kinetics of crude oils—thermogravimetry approach. J Pet Sci Eng. 2018;167:480–5.
Kok MV, Pokol G, Keskin C, Madarasz J, Bagci S. Combustion characteristics of lignite and oil shale samples by thermal analysis techniques. J Therm Anal Calorim. 2004;76(1):247–54.
**n HH, Wang DM, Qi XY, Zhong XX, Ma LY, Dou GL, et al. Oxygen consumption and chemisorption in low-temperature oxidation of sub-bituminous pulverized coal. Spectr Lett. 2018;51(2):104–11.
Simon P. Isoconversional methods—fundamentals, meaning and application. J Therm Anal Calorim. 2004;76(1):123–32.
Kim HS, Yang HS, Kim HJ, Park HJ. Thermogravimetric analysis of rice husk flour filled thermoplastic polymer composites. J Therm Anal Calorim. 2004;76(2):395–404.
Wang CA, Zhang YH, Wang PQ, Zhang JP, Du YB, Che DF. Effects of silicoaluminate oxide and coal blending on combustion behaviors and kinetics of Zhundong coal under oxy-fuel condition. J Therm Anal Calorim. 2018;134(3):1975–86.
Wang CA, Zhang XM, Liu YH, Che DF. Pyrolysis and combustion characteristics of coals in oxyfuel combustion. Appl Energy. 2012;97:264–73.
Pu G, Lei Q, Xu P. Thermogravimetric study on combustion and kinetic characteristics of artificial-plates. Chin J Environ Eng. 2012;6(7):2431–6.
Raveendran K, Ganesh A, Khilar KC. Pyrolysis characteristics of biomass and biomass components. Fuel. 1996;75(8):987–98.
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut. 2011;159(12):3269–82.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881.
Flynn JH, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci Part B-Polym Lett. 1966;4(5PB):323.
Ma ZQ, Chen DY, Gu J, Bao BF, Zhang QS. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers Manage. 2015;89:251–9.
Aboulkas A, El Harfi K, El Bouadili A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: pyrolysis kinetics and mechanisms. Energy Convers Manage. 2010;51(7):1363–9.
Wang CA, Liu YH, Zhang XM, Che DF. A study on coal properties and combustion characteristics of blended coals in Northwestern China. Energy Fuels. 2011;25(8):3634–45.
Wang CA, Liu YH, ** X, Che DF. Effect of water washing on reactivities and NOx emission of Zhundong coals. J Energy Inst. 2016;89(4):636–47.
Jayaraman K, Kok MV, Gokalp I. Combustion properties and kinetics of different biomass samples using TG-MS technique. J Therm Anal Calorim. 2017;127(2):1361–70.
Kok MV, Ozgur E. Thermal analysis and kinetics of biomass samples. Fuel Process Technol. 2013;106:739–43.
Ren X, Chen J, Li G, Wang Y, Lang X, Fan S. Thermal oxidative degradation kinetics of agricultural residues using distributed activation energy model and global kinetic model. Bioresour Technol. 2018;261:403–11.
Jiang G, Wei L, Teng H, Hao H. A kinetic model based on TGA data for pyrolysis of Zhundong coal. CIESC. 2017;68(4):1415–22.
Yuan H, Liu R, Jiang H, Hao Y. Kinetic study of sunflower seed husk pyrolysis. Trans Chin Soc Agric Eng. 2006;22(4):220–3.
Li X, Lin Q. Maximum probability mechanisms of pyrolysis of corn stalk. CIESC. 2012;63(8):2599–605.
Zhang JJ, Ren N. A new kinetic method of processing TA data. Chin J Chem. 2004;22(12):1459–62.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions—a variant on the Ozawa–Flynn–Wall method. Thermochim Acta. 1996;285(2):309–23.
Bohn MA. Problems and faulty uses with the Prout–Tompkins description of autocatalytic reactions and the solutions. J Therm Anal Calorim. 2014;116(2):1061–72.
Niu SB, Chen M, Li Y, Song J. Co-combustion characteristics of municipal sewage sludge and bituminous coal. J Therm Anal Calorim. 2018;131(2):1821–34.
Zhang J, Xue BB, Rao GN, Chen LP, Chen WH. Thermal decomposition characteristic and kinetics of DINA. J Therm Anal Calorim. 2018;133(1):727–35.
Li D, Chen L, Yi X, Zhang X, Ye N. Pyrolytic characteristics and kinetics of two brown algae and sodium alginate. Bioresour Technol. 2010;101(18):7131–6.
Acknowledgements
The authors acknowledged the financial support from the China Postdoctoral Science Foundation (2019T120286), the State Key Laboratory of Pollution Control and Resource Reuse Foundation (PCRRF18009), and the Natural Science Basic Research Plan in Shaanxi Province of China (2019JM-067).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., **, L., Wang, Y. et al. Thermogravimetric investigation on co-combustion characteristics and kinetics of antibiotic filter residue and vegetal biomass. J Therm Anal Calorim 147, 925–938 (2022). https://doi.org/10.1007/s10973-020-10280-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10280-2