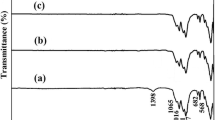
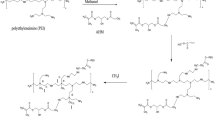
Abstract
Demand of zirconia based bone implant substitutes is increasing day after day as it creates a pathogen free environment along with healing and osseointegration. A big problem associated with zirconia is its stability as its biocompatibility and mechanical properties alter from stabilized tetragonal zirconia to monoclinic leading towards the poor cosmesis. Present work’s aim is preparation of stabilized zirconia by employing microwave (MW) assisted sol–gel technique. Honey is used to prevent particles from hard agglomeration that acted as cap** agent. Effect of microwave powers on zirconia stabilization is observed in the range of 100–1000 W. Low microwave values of 100 and 200 W lead to formation of pure phase of t-ZrO2. High hardness value (~1510 HV) is observed at 100 W with fracture toughness ~28.80 MPam−1/2. Binding energy values of Zr3d3/2 and Zr3d5/2 are observed at 185.33 eV and 183.92 eV, respectively. Vibrating sample magnetometer results show the superparamagnetic behavior for optimized sample which is well suited for biological applications. Pure phase zirconia prepared using 100 W microwave power shows relatively higher value of dielectric constant (~73) and low tangent loss. Biodegradation studies show smaller values of weight loss even after immersion in simulated body fluid (SBF) after 26 weeks. Antibacterial activity shows highest zone of inhibition (~33 mm) against E.coli bacteria. Honey mediated zirconia shows high anti-oxidant activity. Thus, honey mediated zirconia can be successfully employed for bone implants.

Synthesis of zirconia is done by means of microwave powers. ZrOCl2.8H2O and de-ionized water were used as starters. Afterwards, honey was added that acted as cap** agent. Obtained sol was further dried in microwave reactor by varying microwave powers. These obtained samples can be used as bone implant as they can inhibit the growth of reactive oxygen species (ROS) and cure inflammations and have tendency to protect bone from further bacterial attacks and damages.


















Similar content being viewed by others
References
Kawai, Y, Uo, M, Wang, Y, Kono, S, Ohnuki, S, & Watari, F (2011). Phase transformation of zirconia ceramics by hydrothermal degradation. Dental Mater J, 1105140139–1105140139.
Tosiriwatanapong T, Singhatanadgit W (2018) Zirconia-based biomaterials for hard tissue reconstruction. Bone Tissue Regeneration Insights 9:1179061X18767886
Hajizadeh-Oghaz M, Razavi RS, Khajelakzay M (2015) Optimizing sol–gel synthesis of magnesia-stabilized zirconia (MSZ) nanoparticles using Taguchi robust design for thermal barrier coatings (TBCs) applications. J Sol-Gel Sci Technol 73(1):227–241
Chevalier J (2006) What future for zirconia as a biomaterial? Biomaterials 27(4):535–543
Shi Q, Yuan W, Chao X, Zhu Z (2017) Phase stability, thermal conductivity and crystal growth behaviour of RE2O3 (RE= La, Yb, Ce, Gd) co-doped Y2O3 stabilized ZrO2 powder. J Sol-Gel Sci Technol 84(2):341–348
Li X, Shimizu Y, Pyatenko A, Wang H, Koshizaki N (2012) Tetragonal zirconia spheres fabricated by carbon-assisted selective laser heating in a liquid medium. Nanotechnology 23(11):115602
Gupta TK, Bechtold JH, Kuznicki RC, Cadoff LH, Rossing BR (1977) Stabilization of tetragonal phase in polycrystalline zirconia. J Mater Sci 12(12):2421–2426
El-Kased RF, Amer RI, Attia D, Elmazar MM (2017) Honey-based hydrogel: in vitro and comparative in vivo evaluation for burn wound healing. Sci Rep. 7(1):1–11
Philip D (2009) Honey mediated green synthesis of gold nanoparticles. Spectrochimica Acta Part A: Mol Biomolecular Spectrosc 73(4):650–653
Philip D (2010) Honey mediated green synthesis of silver nanoparticles. Spectrochimica Acta Part A: Mol Biomolecular Spectrosc 75(3):1078–1081
Venu R, Ramulu TS, Anandakumar S, Rani VS, Kim CG (2011) Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf A: Physicochemical Eng Asp 384(1-3):733–738
Reddy SM, Datta KKR, Sreelakshmi C, Eswaramoorthy M, Reddy BV (2012) Honey mediated green synthesis of Pd nanoparticles for suzuki coupling and hydrogenation of conjugated olefins. Nanosci Nanotechnol Lett 4(4):420–425
Heshmatpour F, Aghakhanpour RB (2011) Synthesis and characterization of nanocrystalline zirconia powder by simple sol–gel method with glucose and fructose as organic additives. Powder Technol 205(1-3):193–200
Ghelich R, Aghdam RM, Sadat Torknik F, Jahannama MR (2018) Synthesis and characterization of biocompatible zirconia nanofibers based on electrospun PVP/Zr (OPr)4. Mater Today: Proc 5(7):15733–15738
Piticescu RR, Monty C, Taloi D, Motoc A, Axinte S (2001) Hydrothermal synthesis of zirconia nanomaterials. J Eur Ceram Soc 21(10-11):2057–2060
Thenmozhi S, Dharmaraj N, Kadirvelu K, Kim HY (2017) Electrospun nanofibers: New generation materials for advanced applications. Mater Sci Eng: B 217:36–48
Azad AM (2006) Fabrication of yttria-stabilized zirconia nanofibers by electrospinning. Mater Lett 60(1):67–72
Siddiqui MRH, Al-Wassil AI, Al-Otaibi AM, Mahfouz RM (2012) Effects of precursor on the morphology and size of ZrO2 nanoparticles, synthesized by sol-gel method in non-aqueous medium. Mater Res 15:986–989
Xu X, Guo G, Fan Y (2010) Fabrication and characterization of dense zirconia and zirconia-silica ceramic nanofibers. J Nanosci Nanotechnol 10(9):5672–5679
Zhao Y, Tang Y, Guo Y, Bao X (2010) Studies of electrospinning process of zirconia nanofibers. Fibers Polym 11(8):1119–1122
Manjunatha S, Dharmaprakash MS (2016) Microwave assisted synthesis of cubic Zirconia nanoparticles and study of optical and photoluminescence properties. J Lumin 180:20–24
Bukhari BS, Imran M, Bashir M, Riaz S, Naseem S (2018) Honey mediated microwave assisted sol–gel synthesis of stabilized zirconia nanofibers. J Sol-Gel Sci Technol 87(3):554–567
Bukhari SB, Imran M, Bashir M, Riaz S, Naseem S (2018) Room temperature stabilized TiO2 doped ZrO2 thin films for teeth coatings–A sol-gel approach. J Alloy Compd 767:1238–1252
Das VK, Das S, Thakur AJ (2012) Protection and deprotection chemistry catalyzed by zirconium oxychloride octahydrate (ZrOCl2· 8H2O). Green Chem Lett Rev 5(4):577–586
Yousaf H, Azhar M, Bashir M, Riaz S, Kayani ZN, Naseem S (2020) Effect of cap** agent on microwave assisted sol-gel synthesized zirconia coatings for optical applications. Optik 222:165297
Palmero P, Montanaro L, Reveron H, Chevalier J (2014) Surface coating of oxide powders: A new synthesis method to process biomedical grade nano-composites. Materials 7(7):5012–5037
Koo JY, Hwang S, Ahn M, Choi M, Byun D, Lee W (2016) Controlling the diameter of electrospun Yttria‐stabilized zirconia nanofibers. J Am Ceram Soc 99(9):3146–3150
Panapoy M, Ksapabutr B (2008) Fabrication of Zirconia nanofibers using Zirconatrane synthesized by Oxide One-Pot Process as precursor. Adv Mater Res 55:605–608
Chattopadhyay S, Bysakh S, Saha J, De G (2018) Electrospun ZrO2 nanofibers: precursor controlled mesopore ordering and evolution of garland-like nanocrystal arrays. Dalton Trans 47(16):5789–5800
Parera JM (1992) Promotion of zirconia acidity by addition of sulfate ion. Catal today 15(3-4):481–490
Son JR, Gwon TD, Kim SB (2001) Characterization of zirconium sulfate supported on zirconia and activity for acid catalysis. Bull Korean Chem Soc 22(12):1309–1315
American Society for Testing of Materials (1999) Designation C1327-99 Standard test method for Vickers indentation hardness of advanced ceramics Annual Book of ASTM Standards 15.01. ASTM, Philadelphia
Ong, KCG, & Akbarnezhad, A (2006). Thermal stresses in microwave heating of concrete. Proc. 31st Our World in Concrete and Structures, 297–310.
Sun J, Wang W, Yue Q (2016) Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 9(4):231
Cullity, BD (1956). Elements of X-ray Diffraction. Addison-Wesley Publishing, New York, NY, USA.
Garvie RC (1965) The occurrence of metastable tetragonal zirconia as a crystallite size effect. J Phys Chem 69(4):1238–1243
Tyagi B, Sidhpuria K, Shaik B, Jasra RV (2006) Synthesis of nanocrystalline zirconia using sol− gel and precipitation techniques. Ind Eng Chem Res 45(25):8643–8650
Riaz S, Ashraf R, Akbar A, Naseem S (2014) Microwave assisted iron oxide nanoparticles—structural and magnetic properties. IEEE Trans Magn 50(8):1–4
Majid F, Mirza ST, Riaz S, Naseem S (2015) Sol-gel synthesis of BiFeO3 nanoparticles. Mater Today: Proc 2(10):5293–5297
Guazzato M, Albakry M, Ringer SP, Swain MV (2004) Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent Mater 20(5):449–456
Guazzato, M, Albakry, M, Swain, MV, & Ironside, J (2002). Mechanical properties of In-Ceram Alumina and In-Ceram Zirconia. International Journal of Prosthodontics, 15(4).
Li, JC (Ed.). (2011). Mechanical properties of nanocrystalline materials. CRC Press.
Niihara K, Morena R, Hasselman DPH (1982) Evaluation of K Ic of brittle solids by the indentation method with low crack-to-indent ratios. J Mater Sci Lett 1(1):13–16
McArthur, SL, Mishra, G, & Easton, CD (2014). Applications of XPS in biology and biointerface analysis. In Surface analysis and techniques in biology, Springer, Cham, 9–36.
Bashir M, Riaz S, Kayani ZN, Naseem S (2018) Synthesis of bone implant substitutes using organic additive based zirconia nanoparticles and their biodegradation study. J Mech Behav Biomed Mater 88:48–57
Park JY, Heo JK, Kang YC (2010) The properties of RF sputtered zirconium oxide thin films at different plasma gas ratio. Bull Korean Chem Soc 31(2):397–400
Lackner P, Zou Z, Mayr S, Diebold U, Schmid M (2019) Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys Chem Chem Phys 21(32):17613–17620
Batool T, Bukhari BS, Riaz S, Batoo KM, Raslan EH, Hadi M (2020) Microwave assisted sol-gel synthesis of bioactive zirconia nanoparticles–correlation of strength and structure. J Mech Behav Biomed Mater 112:104012
Gabriel S, Lau RW, Gabriel C (1996) The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol 41(11):2251
Pethig R (1985) Dielectric and electrical properties of biological materials. J Bioelectr 4(2):vii–ix
Lazebnik M, Converse MC, Booske JH, Hagness SC (2006) Ultrawideband temperature-dependent dielectric properties of animal liver tissue in the microwave frequency range. Phys Med Biol 51(7):1941
Ji Z, Brace CL (2011) Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys Med Biol 56(16):5249
Sanaullah I, Imran M, Riaz S, Amin T, Khan IU, Zahoor R, Naseem S (2021) Microwave assisted synthesis of Fe3O4 stabilized ZrO2 nanoparticles–Free radical scavenging, radiolabeling and biodistribution in rabbits. Life Sci 271:119070
Macdonald JR, Barsoukov E (2005) Impedance spectroscopy: theory, experiment, and applications. History 1(8):1–13
Sharma HB, Devi KN, Gupta V, Lee JH, Singh SB (2014) Ac electrical conductivity and magnetic properties of BiFeO3–CoFe2O4 nanocomposites. J Alloy Compd 599:32–39
Quartarone E, Mustarelli P, Magistris A (1998) PEO-based composite polymer electrolytes. Solid State Ion 110(1-2):1–14
Jha AK (2013) Electrical characterization of zirconium substituted barium titanate using complex impedance spectroscopy. Bull Mater Sci 36(1):135–141
Das R, Sarkar T, Mandal K (2012) Multiferroic properties of Ba2+ and Gd3+ co-doped bismuth ferrite: magnetic, ferroelectric and impedance spectroscopic analysis. J Phys D: Appl Phys 45(45):455002
Verma KC, Ram M, Singh J, Kotnala RK (2011) Impedance spectroscopy and dielectric properties of Ce and La substituted Pb0.7Sr0.3 (Fe0.012Ti0.988) O3 nanoparticles. J Alloy Compd 509(15):4967–4971
Imran Z, Rafiq MA, Ahmad M, Rasool K, Batool SS, Hasan MM (2013) Temperature dependent transport and dielectric properties of cadmium titanate nanofiber mats. AIP Adv 3(3):032146
Miller JS (2011) Magnetically ordered molecule-based materials. Chem Soc Rev 40(6):3266–3296
Miller JS, Epstein AJ (1994) Organic and organometallic molecular magnetic materials—designer magnets. Angew Chem Int Ed Engl 33(4):385–415
Ovcharenko VI, Sagdeev RZ (1999) Molecular ferromagnets. Russian Chem Rev 68(5):345–363
Liu H, Yang J, Zhang Y, Yang L, Wei M, Ding X (2009) Structure and magnetic properties of Fe-doped ZnO prepared by the sol–gel method. J Phys: Condens Matter 21(14):145803
Rasouli E, Basirun WJ, Rezayi M, Shameli K, Nourmohammadi E, Khandanlou R, Sarkarizi HK (2018) Ultrasmall superparamagnetic Fe3O4 nanoparticles: honey-based green and facile synthesis and in vitro viability assay. Int J Nanomed 13:6903
Pattnaik S, Nethala S, Tripathi A, Saravanan S, Moorthi A, Selvamurugan N (2011) Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. Int J Biol macromolecules 49(5):1167–1172
Ochsner, A, & Altenbach, H (Eds.). (2013). Advances in bio-mechanical systems and materials. Springer International Publishing Switzerland 2013.
Sohrabi M, Eftekhari Yekta B, Rezaie H, Naimi-Jamal MR, Kumar A, Cochis A, Miola M, Rimondini L (2020) Enhancing mechanical properties and biological performances of injectable bioactive glass by gelatin and chitosan for bone small defect repair. Biomedicines 8(12):616
Katsanevakis E, Wen XJ, Shi DL, Zhang N (2010) Biomineralization of polymer scaffolds. Key Eng Mater 441:269–295
Koutouzis T, Wallet S, Calderon N, Lundgren T (2011) Bacterial colonization of the implant–abutment interface using an in vitro dynamic loading model. J Periodontol 82(4):613–618
Martinotti S, Bucekova M, Majtan J, Ranzato E (2019) Honey: an effective regenerative medicine product in wound management. Curr Medicinal Chem 26(27):5230–5240
Al-Brahim JS, Mohammed AE (2020) Antioxidant, cytotoxic and antibacterial potentials of biosynthesized silver nanoparticles using bee’s honey from two different floral sources in Saudi Arabia. Saudi. J Biol Sci 27:363–373
Neupane BP, Chaudhary D, Paudel S, Timsina S, Chapagain B, Jamarkattel N, Tiwari BR (2019) Himalayan honey loaded iron oxide nanoparticles: Synthesis, characterization and study of antioxidant and antimicrobial activities. Int J Nanomed 14:3533
Bonsignore G, Patrone M, Martinotti S, Ranzato E (2021) “Green” Biomaterials: The Promising Role of Honey. J Funct Biomater 12(4):72
Balasooriya ER, Jayasinghe CD, Jayawardena UA, Ruwanthika RWD, Mendis de Silva R, Udagama PV (2017) Honey mediated green synthesis of nanoparticles: new era of safe nanotechnology. J Nanomater 2017:10
Al-Brahim JS, Mohammed AE (2020) Antioxidant, cytotoxic and antibacterial potentials of biosynthesized silver nanoparticles using bee’s honey from two different floral sources in Saudi Arabia. Saudi J Biol Sci 27(1):363–373
Khalil MI, Sulaiman SA, Boukraa L (2010) Antioxidant properties of honey and its role in preventing health disorder. Open Nutraceuticals J 3(1):6–16
Golestanzadeh M, Naeimi H, Zahraie Z (2017) Synthesis and antioxidant activity of star-shape phenolic antioxidants catalyzed by acidic nanocatalyst based on reduced graphene oxide. Mater Sci Eng: C 71:709–717
Balaji S, Mandal BK, Ranjan S, Dasgupta N, Chidambaram R (2017) Nano-zirconia–evaluation of its antioxidant and anticancer activity. J Photochemistry Photobiol B: Biol 170:125–133
Ferreres F, Ortiz A, Silva C, Garcia-Viguera C, Tomás-Barberán FA, Tomás-Lorente F (1992) Flavonoids of “La Alcarria” honey A study of their botanical origin. Z für Lebensm-Unters und Forsch 194(2):139–143
Andrade P, Ferreres F, Amaral MT (1997) Analysis of honey phenolic acids by HPLC, its application to honey botanical characterization. J Liq Chromatogr Relat Technol 20(14):2281–2288
Acknowledgements
The authors thank Higher Education Commission Pakistan for providing financial support. The authors thank Dr. Sajid ur Rehman for providing XPS analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
SanaUllah, I., Khan, H.N., Saleha, M. et al. Free radical scavenging and antimicrobial activities of MW assisted sol-gel synthesized honey mediated zirconia. J Sol-Gel Sci Technol 103, 457–475 (2022). https://doi.org/10.1007/s10971-022-05817-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05817-w