Abstract
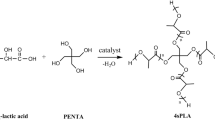
Poly (L-lactic acid) (PLLA) is a bio-based biodegradable polymer with minimal immunogenicity, non-toxicity and excellent mechanical properties and has widely used in medical, pharmaceutical, food packaging and other fields. However, linear PLLA has the characteristics of high melting point, strong crystallinity, weak flowability and poor thermal stability, which cannot meet the requirements of various industrial processes and novel pharmaceutical formulations. With its low viscosity, controlled degradation cycle, and excellent melt flowability, star-shaped PLLA can be used in the fields of biocomposites, biomedical devices, drug delivery, and intelligent packaging. Presently, metal catalysts and organic solvents are frequently used in PLLA synthesis industries, and both residues continue to pose a safety risks. In existing studies, the performance of bioenzymes as optimal green catalysts in organic solvents is not satisfactory, and they are predominantly used to synthesise oligomers with low yields and lengthy reaction times. In this study, a four-armed star-shaped poly (L-lactic acid) (4s-PLLA) with a porous structure favourable for drug loading was synthesised using supercritical carbon dioxide (Sc-CO2) as a solvent, lipase as a catalyst, and erythritol as a nucleus. Using Sc-CO2 as a solvent the reaction activity and stability of the enzyme were increased so that the reaction yield could reach 83% and the reaction time was only 24 h, which provided a possibility for the practical application of the enzymes catalyze green process. Both the random copolymerization product four-armed star-shaped poly(L-lactic-glycolic acid) (4s-PLGA) and the block copolymerization product four-armed star-shaped poly (L-lactic-glycolic acid) (4s-PLLA-PGA) were subsequently synthesized by copolymerization modification, and their porous structures retained and hydrophilicity were enhanced. The response surface analyses were performed about the reaction time, reaction temperature and yield of synthetic reactions by using Design-Expert 12 software and the prediction equations were generated.
















Similar content being viewed by others
Data availability
Authors can confirm that all relevant data are included in the article.
References
Ilyas R, Zuhri M, Aisyah H et al (2022) Natural fiber-reinforced polylactic acid, polylactic acid blends and their composites for advanced applications. Polymers 14(1):202–202. https://doi.org/10.3390/polym14010202
J-C W XL et al (2022) Synthesis of branched poly(lactic acid) and its utilization in the biomedical field. Plast Sci Technol 006:114–118. https://doi.org/10.15925/j.cnki.issn1005-3360.2022.06.022
Corneillie S, Smet M (2015) PLA architectures: the role of branching. Polym Chem 6(6):850–867. https://doi.org/10.1039/C4PY01572J
Rafiei P, Haddadi A (2019) A robust systematic design: optimization and preparation of polymeric nanoparticles of plga for docetaxel intravenous delivery. Mater Sci Engineering: C 104:109950. https://doi.org/10.1016/j.msec.2019.109950
Sun, Yu et al (2021) Synthesis and characterizations of gentamicin-loaded poly-lactic-co-glycolic (PLGA) nanoparticles. J Nanopart Res 23(8):1–15. https://doi.org/10.1007/s11051-021-05293-3
Danhier F, Ansorena E, Silva JM et al (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Controlled Release 161(2):505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
Adam, Kowalski et al (2000) Polymerization of l, l-Dilactide initiated by tin(II) Butoxide. Macromolecules 33(6):1964–1971. https://doi.org/10.1021/MA991751S
Mena, María et al (2010) Enzymatic synthesis of poly-L-lactide-co-glycolide in the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. Bioprocess Biosyst Eng 33(9):1095–1101. https://doi.org/10.1007/s00449-010-0435-5
Thurecht K J H S M (2009) Controlled dispersion polymerization in supercritical Carbon Dioxide. Aust J Chem 62(8):786–789. https://doi.org/10.1071/CH09081
Yoon SeungKyun and Chung DongJune (2022) In vivo degradation studies of PGA-PLA Block Copolymer and their histochemical analysis for spinal-fixing application. Polymers 14(16):3322–3322. https://doi.org/10.3390/polym14163322
Uyama H, Kobayashi S (2019) Synthesis of Polyesters II: hydrolase as Catalyst for Ring-Opening Polymerization. Singapore:enzymatic polymerization towards. Green Polym Chem 37(10). https://doi.org/10.1007/978-981-13-3813-7_6
Hans Marc K, Helmut, Moeller Martin (2009) Ring-opening polymerization of DD-lactide catalyzed by Novozyme 435. Macromol Biosci 9(3):239–247. https://doi.org/10.1002/mabi.200800236
Zhao Hua (2019) Enzymatic polymerization to polyesters in nonaqueous solvents. Methods Enzymol 627:1–21. https://doi.org/10.1016/bs.mie.2019.03.002
Di Martino A, Trusova ME, Postnikov PS et al (2018) Branched poly (lactic acid) microparticles for enhancing the 5-aminolevulinic acid phototoxicity. J Photochem Photobiol B 181:80–88. https://doi.org/10.1016/j.jphotobiol.2018.03.001
Huang D, Wu D (2018) Biodegradable dendrimers for drug delivery. Mater Sci Eng C Mater Biol Appl 90:713–727. https://doi.org/10.1016/j.msec.2018.03.002
Nizamuddin S, Jadhav A, Qureshi SS et al (2019) Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci Rep 9(1):5445. https://doi.org/10.1038/s41598-019-41960-1
Choubisa B, Patel M, Dholakiya B (2012) Synthesis and characterization of polylactic acid (PLA) using a solid acid catalyst system in the polycondensation method. Res Chem Intermed 39(7):3063–3070. https://doi.org/10.1080/10601325.2013.802157
Ding L, ** W, Chu Z et al (2011) Bulk solvent-free melt ring-opening polymerization (ROP) of L-lactide catalyzed by Ni(II) and ni(II)-Ln(III) complexes based on the acyclic salen-type Schiff-base ligand. Inorg Chem Commun 14(8):1274–1278. https://doi.org/10.1016/j.inoche.2011.04.040
Prajapati SK, Jain A, Jain A et al (2019) Biodegradable polymers and constructs: a novel approach in drug delivery. Eur Polymer J 120:109191. https://doi.org/10.1016/j.eurpolymj.2019.08.018
Zhang S-Y, Chen Z-F, Wu F et al (2016) The molecular weight dependence of the crystallization behavior of four-arm poly(L-lactide). Colloid Polym Sci 294(11):1865–1870. https://doi.org/10.1007/s00396-016-3936-1
Zhan S-P, Huang X, Zhao Q-C et al (2013) A high efficiency PDMS-Based stabilizer for dispersion polymerization of L-lactide in supercritical Carbon Dioxide. J Macromolecular Sci Part A 50(10):1070–1074. https://doi.org/10.1080/10601325.2013.821916
Xueyong Y et al (2019) Polylactide-based chiral porous monolithic materials prepared using the High Internal Phase Emulsion Template Method for Enantioselective Release. ACS Biomater Sci Eng 5:5072–5081. https://doi.org/10.1021/acsbiomaterials.9b01276
Gao A, Zhao Y, Yang Q et al (2016) Facile preparation of patterned petal-like PLA surfaces with tunable water micro-droplet adhesion properties based on stereo-complex co-crystallization from non-solvent induced phase separation processes. J Mater Chem A 4(31):12058–12064. https://doi.org/10.1039/C6TA02794F
Surya N, Bhattacharyya S (2021) PLGA-ПЕРСПЕКТИВНЫЙ ПОЛИМЕР ДЛЯДОСТАВКИ ЛЕКАРСТВЕННЫХ СРЕДСТВ. Pharm Pharmacol 9(5):334–345. https://doi.org/10.19163/2307-9266-2021-9-5-334-345
Liu H, Webster TJT (2010) Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int J Nanomed 5(1):299–313. https://doi.org/10.2147/IJN.S9882
Grzegorz Lapienis (2009) Star-shaped polymers having PEO arms. Prog Polym Sci 34(9):852–892. https://doi.org/10.1016/jprogpolymsci200904006
Mei Lin,Jiang Yuyang,Feng Si-Shen (2014) Star-shaped block polymers as a molecular biomaterial for nanomedicine development. Nanomed (London Engl 9(1):9–12. https://doi.org/10.2217/nnm.13.180
Chou SF, Woodrow KA (2017) Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J Mech Behav Biomed Mater 65:724–733. https://doi.org/10.1016/j.jmbbm.2016.09.004
Enas M, Elmowafy, Tiboni M, Mahmoud E, Soliman (2019) Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Invest 49(4):347–380. https://doi.org/10.1007/s40005-019-00439-x
Netti PA, Biondi M, Frigione M (2020) Experimental studies and modeling of the degradation process of poly(Lactic-co-Glycolic Acid) microspheres for sustained protein release. Polymers 12(9):1–16. https://doi.org/10.3390/polym12092042
Daniel J, Hines, Kaplan DL (2013) Poly(lactic-co-glycolic) acid – controlled-release Systems: experimental and modeling insights. Crit Rev Ther Drug Carr Syst 30(3):257–276. https://doi.org/10.1615/critrevtherdrugcarriersyst.2013006475
Haggag Yusuf A et al (2018) Effect of poly(ethylene glycol) content and formulation parameters on particulate properties and intraperitoneal delivery of insulin from PLGA nanoparticles prepared using the double-emulsion evaporation procedure. Pharm Dev Technol 23(4):370–381. https://doi.org/10.1080/10837450.2017.1295066
Piergiorgio G et al (2014) An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. IJMS 15(3):3640–3659. https://doi.org/10.3390/ijms15033640
Author information
Authors and Affiliations
Ethics declarations
Conflict of interests
The authors declare no confict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhan, S. Synthesis of star poly (L-lactic acid) and its copolymers catalyzed by lipase in supercritical carbon dioxide. J Polym Res 30, 414 (2023). https://doi.org/10.1007/s10965-023-03777-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03777-5