Abstract
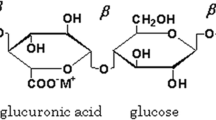
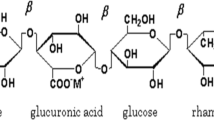
Solid electrolyte for electrochemical device applications have been developed using a linear anionic polysaccharide, Gellan gum incorporated with Ammonium formate (NH4HCO2) by the solution casting technique using double distilled water as solvent. The amorphous nature and the crystallinity percentage of the polymer membranes have been calculated from X-ray Diffraction (XRD) technique and the complex formation between the polymer and salt have been studied using Fourier transform infrared (FTIR) technique. Ionic conductivity of developed membranes has been found by measuring its impedance. Polymer membrane (1 g Gellan gum: 0.9 M.wt % of NH4HCO2) exhibits the conductivity of 5.62 ± 0.09 × 10–3 S/cm. The Differential Scanning Calorimetric (DSC) thermograms have been used to study the glass transition temperature in the membranes. The predominant transportation of ions has been proved by DC Wagner’s Polarization technique. The electrochemical stability for the highest ion conducting polymer membrane has been studied using Linear sweep Voltametry (LSV). Using highest conducting polymer membrane as an electrolyte, the electrochemical devices – primary battery, rechargeable battery and proton exchange membrane (PEM) fuel cell have been constructed and their performance has been analyzed. The primary battery exhibited the open circuit voltage (OCV) of 1.78 V, the rechargeable battery provided the highest potential of 2.27 V and the PEM fuel cell exhibits the OCV of 763 mV.








Similar content being viewed by others
References
Subba Reddy CV, Sharmanad AK, Narasimha Rao VVR (2004) Characterization of a solid state battery based on polyblend of (PVP+PVA+KBr O3) electrolyte. Ionics 10:142–147
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state aer like polymer super capacitors. Nano Lett 10:4025–4403
Fang J, Qiao J, Wilknson DP, Zhang J (eds) (2015) Electrochemical polymer electrolyte membranes. CRC Press, New York
Hassan M, Azimi N (2019) Conductivity and transport properties of starch/glycerin-MgSO4 solid polymer electrolytes. Int J Adv Appl Sci 6:38–43
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2019) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25:2147–2157
Naachiyar R, Ragam M, Selvasekarapandian S, Krishna M, Buvaneshwari P (2021) Development of biopolymer electrolyte membrane using Gellan gum biopolymer incorporated with NH4SCN for electro-chemical application. Ionics 27:3415–3429
Vanitha N, Shanmugapriya C, Subramanian S, Krishna M, Vengadesh N, Karuppasamy, (2022) Investigation of N-S-based graphene quantum dot on sodium alginate with ammonium thiocyanate (NH4SCN) biopolymer electrolyte for the application of electrochemical devices. J Mater Sci Mater Electron 33:1–21
Majid SR, Sabadini RC, Kanicki J, Pawlicka A (2014) Impedance analysis of gellan gum-poly (vinyl pyrrolidone) membranes. Mol Cryst Liq Cryst 604(1):84–95
Noor IM, Majid SR, Arof AK, Djurado D, Neto SC, Pawlicka A (2012) Characteristics of gellan gum–LiCF3SO3 polymer electrolytes. Solid State Ion 225:649–653
Noor IM (2020) Determination of charge carrier transport properties of gellan gum–lithium triflate solid polymer electrolyte from vibrational spectroscopy. High Perform Polym 32:168–174
Neto MJ, Sentanin F, Esperança J, Medeiros MJ, Pawlicka A, Bermudez V, Silva M (2015) Gellan gum—ionic liquid membranes for electrochromic device application. Solid State Ion 274
Rahul S, Bhaskar B, Hee-Woo R, Pramod S (2015) Solid gellan gum polymer electrolyte for energy application. Int J Hydrogen Energy 40:9365–9372
Karthika J, Vishalakshi B, Jagadish N (2015) Gellan gum–graft–polyaniline—an electrical conducting biopolymer. Int J Biol Macromol 82:61–67
Abdul Halim NF, Majid SR, Arof AK, Kajzar F, Pawlicka A (2012) Gellan gum-LiI gel polymer electrolytes. Mol Cryst Liq Cryst 554:232–238
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M, Vinoth Pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Moniha V, Marimuthu A, Selvasekarapandian S, Sundaresan B, Hemalatha R (2019) Development and characterization of bio-polymer electrolyte iota-carrageenan with ammonium salt for: electrochemical application. Mater Today Proc 8:449–455
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi S, Aristatil G, Arun A, Saminathan M (2017) Proton-conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23:2775–2780
Boopathi G, Pugalendhi S, Selvasekarapandian S, Premalatha M, Monisha S, Aristatil G (2017) Development of proton conducting biopolymer membrane based on agar–agar for fuel cell. Ionics 23:2781–2790
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2018) A study of electrochemical devices based on Agar-Agar-NH4I biopolymer electrolytes. AIP Conference Proceedings 140019
Selvalakshmi S, Mathavan T, Selvasekarapandian S et al (2019) Characterization of biodegradable solid polymer electrolyte system based on agar-NH4Br and its comparison with NH4I. J Solid State Electrochem 23:1727–1737
Premalatha M, Mathavan T, Selvasekarapandian S, Selvalakshmi S, Monisha S (2017) Incorporation of NH4Br in tamarind seed polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org Electron 50:418–425
Premalatha M, Mathavan T, Selvasekarapandian S, Selvalakshmi S (2018) Structural and electrical characterization of tamarind seed polysaccharide (TSP) doped with NH4HCO2. AIP Conference Proceedings 070005
Mohamed A, Abd. Shukur M, Kadir M, Yusof Y (2020) Ion conduction in chitosan-starch blend based polymer electrolyte with ammonium thiocyanate as charge provider. J Polym Res 27(6)
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37(8):1371–1376
Vinoth Pandi D, Selvasekarapandian S, Bhuvaneswari R, Premalatha M, Monisha S, Arun Kumar D, Kawamura J (2016) Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ion 298:15–22
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Vinoth Pandi D, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Boukamp BA (1986) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion 20(1):31–44
Machado GD, Regiani AM, Pawlicka A (2003) Carboxymethylcellulose derivatives with low hydrophilic properties. Polimery 48(4):273–279
Mollá S, Compañ V (2011) Polyvinyl alcohol nanofiber reinforced Nafion membranes for fuel cell applications. Energy Fuels 372:191–200
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Shanthi G, Sampathkumar L, Maheshwari T (2021) Impact of ammonium formate (AF) and ethylene carbonate (EC) on the structural, transport and electrochemical properties of pectin based biopolymer membranes. Ionics 27:3443–3459
Chenliang Gong Yu, Liang ZQ, Li H, Zhongying Wu, Zhang Z, Zhang S, Zhang X, Li Y (2015) Solution processable octa(aminophenyl)silsesquioxane covalently cross-linked sulfonated polyimides for proton exchange membranes. J Membr Sci 476:364–372
Yang T, Li Z, Lyu H, Zheng J, Liu J, Liu F, Zhang Z, Rao H (2018) A graphene oxide polymer brush based cross-linked nanocomposite proton exchange membrane for direct methanol fuel cells. RSC Adv 8(28):15740–15753
Pasquini L, Zhakisheva B, Sgreccia E, Narducci R, Di Vona ML, Knauth P (2021) Stability of proton exchange membranes in phosphate buffer for enzymatic fuel cell application: Hydration, conductivity and mechanical properties. Polymers (Basel) 13(3):475
Zhao C, Lin H, Shao K, Li X, Ni H, Wang Z, Na H (2006) Block sulfonated poly(ether ether ketone)s (SPEEK) ionomers with high ion-exchange capacities for proton exchange membranes. J Power Sources 162:003–1009
Pandey K, Lakshmi N, Chandra S (1998) A rechargeable solid state proton battery with an intercalating cathode and an anode containing a hydrogen storage-material. J Power Sources 76(1):116–123
Tamilarasan K, Selvasekarapandian S, Chitra R, Kiruthika S (2021) Investigation of blend biopolymer electrolytes based on Dextran-PVA with ammonium thiocyanate. J Solid State Electrochem 25:1–11
Hemalatha R, Marimuthu A, Selvasekarapandian S, Sundaresan B, Moniha V (2019) Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J Sci Adv Mater Dev 4:101–110
Hemalatha R, Alagar M, Selvasekarapandian S, Sundaresan B, Moniha V, Boopathi G, Christopher S (2019) Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. Ionics 25:141–154
Tang Y, Yuan W, Pan M, Wan Z (2010) Feasibility study of porous copper fiber sintered felt: A novel porous flow field in proton exchange membrane fuel cells. Int J Hydrogen Energy 35:9661–9677
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2017) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Hemalatha R, Boopathi G (2018) Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J Solid State Electrochem 22:1–15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
R, M., M, R., S, S. et al. Fabrication of rechargeable proton battery and PEM fuel cell using biopolymer Gellan gum incorporated with NH4HCO2 solid electrolyte. J Polym Res 29, 337 (2022). https://doi.org/10.1007/s10965-022-03190-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03190-4