Abstract
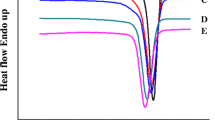
In this study, flexible Nylon-6 was reinforced by semi-or wholly-aromatic polyamides; poly(m-phenylene isophthalamide) (PmIA), poly(4,4′-diphenylmethane terephthalamide) (PMA), and poly(4,4′-diphenylsulfone terephthalamide) (PSA). Various high molecular weight block copolyamides were synthesized by solution polymerization using p-aminophenylacetic acid (p-APA) as a coupling agent. Their thermal properties have shown that the block copolyamides exhibit higher Tg and Tm, and better thermal stability than those of Nylon-6, especially PmIA modified Nylon-6. The order of their thermal properties of aromatic modified Nylon-6 copolyamides is PmIA > PMA > PSA. Moreover, the Tg and Tm of multiblock copolyamides are higher than those of triblock copolyamides. From the wide-angle X-ray diffraction pattern, it is found that the triblock copolyamides have two diffraction peaks, i.e., 2θ = 20.5° and 24°. However, the multiblock has only one peak at 2θ = 20°, indicating a different crystal structure for multiblock copolyamides. For the mechanical properties, it is found that the multiblock copolyamides have a more significant reinforcing effect than the triblock copolyamides. Also, the order of the physical properties of aromatic modified Nylon-6 copolyamides, such as tensile strength, is PmIA > PMA > PSA, but for the elongation PSA > PMA > PmIA.
Similar content being viewed by others
References
M. Takayanagi, T. Ogata, M. Morikawa and T. Kai, J. Macromol. Sci. Phys., B, 17, 591 (1980).
D. R. Moore and L. J. Mathias, J. Appl. Polym. Sci., 32, 6299 (1986).
R. Martin, W. Gotz and B. Vollmert, Angew. Makromol. Chem., 132, 91 (1985).
H. H. Wang and W. L Chen, J. Polym. Sci., Polym. Chem. Ed., 28, 1 (1990).
H. H. Wang and M. F. Lin, J. Appl. Polym. Sci., 43, 259 (1991).
M. F. Lin, H. H. Wang and Y. C. Shu, SAMPE J., 2, 20 (1994).
H. H. Wang and W. L Chen, J. Polym. Sci., Polym. Chem. Ed., 27, 1359 (1989).
Reported in Macromolecular Synthesis, 1, 91 (1977).
O. Olabisi, L. M. Robeson and M. T. Shaw, Polymer-Polymer Miscibility, Academic Press, New York, Chap. 3, 1979.
B. M. Ginzburg, E. T. Magdalev, V. N. Volosatov, R. G. Fedorova, A. M. Shchetinin and S. Ya. Frenkel, Vysokomol. Soedin. B, 22, 660 (1980).
A. Jeziorny, J. Appl. Polym. Sci., 28, 1025 (1983).
D. R. Holmes, C. W. Bunn and D. J. Smith, J. Polym. Sci., Polym. Chem. Ed., 17, 159 (1955).
B. Masar, P. Cefelin and J. Sebenda, Collet. Czech. Chem. Commun., 43, 1983 (1978).
K. Saotome and K. Sato, Makromol. Chem., 102, 105 (1967).
H. Herlinger, H. P. Hoerner, F. Druschke, W. Denneler and F. Haiber, Angew. Makromol. Chem., 29, 229 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, HH., Lin, MF. Modification of nylon-6 with semi-or wholly-aromatic polyamides. J Polym Res 5, 51–58 (1998). https://doi.org/10.1007/s10965-006-0040-0
Issue Date:
DOI: https://doi.org/10.1007/s10965-006-0040-0