Abstract
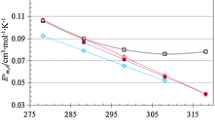
Densities of aqueous solutions of L-glutamic acid and magnesium-L-glutamate were determined from T=288.15 to 333.15 K at 5 K temperature intervals. The measured densities were used to evaluate the apparent molar volumes, V 2,φ (m,T), the cubic expansion coefficients, α(m,T), and the changes of isobaric heat capacities with respect to pressure, (∂ C p /∂ p) T,m . They were qualitatively correlated with changes in the structure of water that occur when L-glutamic acid or magnesium-L-glutamate are present.
Similar content being viewed by others
References
Manzurola, E., Apelblat, A.: Apparent molar volumes of citric, tartaric, malic, succinic, maleic, and acetic acids in water at 298.15 K. J. Chem. Thermodyn. 17, 579–584 (1989)
Apelblat, A., Manzurola, E.: Solubility of ascorbic, 2-furancarboxylic, glutaric, pimelic, salicylic, and o-phthalic acids in water from 279.15 to 342.15 K, and apparent molar volumes of ascorbic, glutaric and pimelic acids in water at 298.15 K. J. Chem. Thermodyn. 21, 1005–1008 (1989)
Apelblat, A., Manzurola, E.: Apparent molar volumes of organic acids and salts in water at 298.15 K. Fluid Phase Equil. 60, 157–171 (1990)
Apelblat, A., Manzurola, E.: Volumetric properties of aqueous solutions of disodium tartrate and L-dipotassium tartrate at temperatures from 278.15 K to 343.15 K and molalities of (0.1, 0.5 and 1.0) mol⋅kg−1. J. Chem. Thermodyn. 33, 1157–1168 (2001)
Orekhova, Z., Ben-Hamo, M., Manzurola, E., Apelblat, A.: Electrical conductance and volumetric studies of aqueous solutions of nicotinic acid. J. Solution Chem. 34, 687–700 (2005)
Orekhova, Z., Sembira, Y., Manzurola, E., Apelblat, A.: Electrical conductance and volumetric studies of aqueous solutions of DL-pyroglutamic acid. J. Solution Chem. 34, 853–867 (2005)
Apelblat, A., Manzurola, E.: Vapour pressure and volumetric studies in aqueous solutions with ascorbate ions. J. Mol. Liquids 131–132, 7–16 (2007)
Greenstein, J.P., Winitz, M.: Chemistry of the Amino Acids. Wiley, New York (1961)
Edsall, J.T., Blanchard, M.H.: The activity ratio of zwitterions and uncharged molecules in ampholyte solutions. The dissociation constants of amino acid esters. J. Am. Chem. Soc. 55, 2353–2337 (1933)
Dalton, J.B., Schmidt, C.L.A.: The solubilities of certain amino acids in water, the densities of their solutions at twenty-five degrees, and the calculated heats of solution and partial molal volumes. J. Biol. Chem. 103, 549–578 (1933)
Millero, F.J., Surdo, A.L., Shin, C.: The apparent molal volumes and adiabatic compressibilities of aqueous amino acids at 25 °C. J. Phys. Chem. 82, 792–784 (1978)
Mishra, A.K., Ahluwalia, J.C.: Apparent molar volumes of amino acids, and peptides in aqueous solutions. J. Phys. Chem. 88, 86–92 (1984)
Rao, M.V.R., Atreyi, M., Rejeswari, M.R.: Partial molar volumes of α−amino acids with ionogenic side chains in water. J. Phys. Chem. 88, 3129–3131 (1984)
Jolicoeur, C., Riedl, B., Desrochers, D., Lemelin, L.L., Zamojska, R., Enea, O.: Solvation of amino acid residues in water and urea-water mixtures: Volumes and heat capacities of 20 amino acids in water and 8 molar urea at 25 °C. J. Solution Chem. 15, 109–128 (1986)
Rao, M.V.R., Atreyi, M., Rejeswari, M.R.: Specific interactions between amino acid side chains—a partial molar volume study. Can. J. Chem. 66, 487–490 (1988)
Hakin, A.W., Duke, M.M., Marty, J.L., Preuss, K.E.: Some thermodynamic properties of aqueous amino acid systems at 288.15, 298.15, 313.15 and 328.15 K: Group additivity analysis of standard-state volumes and heat capacities. J. Chem. Soc., Faraday Trans. 90, 2027–2035 (1994)
Yasuda, Y., Tochio, N., Sakurai, M., Nitta, K.: Partial molar volumes and isentropic compressibilities of amino acids in dilute aqueous solutions. J. Chem. Eng. Data 43, 205–214 (1998)
Banipal, T.S., Kapoor, P.: Partial molal volumes and expansibilities of some amino acids in aqueous solutions. J. Indian Chem. Soc. 76, 431–437 (1999)
Häckel, M., Hinz, H.J., Hedwig, G.R.: Partial molar volumes of proteins: amino acid side-chain contributions derived from the partial molar volumes of some tripeptides over the temperature range 10–90 °C. Biophys. Chem. 82, 35–50 (1999)
Ziemer, S.P., Woolley, E.M.: Thermodynamics of the first and second proton dissociations from aqueous L-aspartic acid and L-glutamic acid at temperatures from (278.15 to 393.15) K and at the pressure 0.35 MPa; Apparent molar heat capacities and apparent molar volumes of zwitterionic, protonated cationic and deprotonated anionic forms at molalities from (0.002 to 1.0) mol⋅kg−1. J. Chem. Thermodyn. 39, 645–666 (2007)
Apelblat, A., Manzurola, E.: Solubilities of L-aspartic, DL-aspartic, DL-glutamic, p-hydroxybenzoic, o-anisic, p-anisic, and itaconic acids in water from 278 K to 345 K. J. Chem. Thermodyn. 29, 1527–1533 (1997)
Yalkovsky, S.H., He, Y.: Aqueous Solubility Data. CRC Press, Boca Raton (2003)
Apelblat, A., Manzurola, E.: Volumetric properties of water, and solutions of sodium chloride and potassium chloride at temperatures from T=277.15 K to 343.15 K at molalities of (0.1, 0.5 and 1.0) mol⋅kg−1. J. Chem. Thermodyn. 31, 869–893 (1999)
Apelblat, A., Manzurola, E.: Volumetric properties of aqueous solutions of lithium chloride from 278.15 K to 338.15 K and molalities of (0.1, 0.5 and 1.0) mol⋅kg−1. J. Chem. Thermodyn. 33, 1133–1155 (2001)
Hepler, L.G.: Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 47, 4613–4617 (1969)
Apelblat, A., Manzurola, E.: Volumetric and thermal properties of some aqueous electrolyte solutions. Part 5. Potassium bromide and potassium iodide 0.1, 0.5, and 1.0 mol⋅kg−1 solutions at temperatures from 278.15 K to 338.15. J. Mol. Liquids 118, 77–88 (2005)
Millero, F.J.: The partial molar volumes of electrolytes in aqueous solutions. In: Horne, R.A. (ed.) Water and Aqueous Solutions. Wiley-Interscience, New York (1972)
Kavanau, J.L.: Water and Solute-Water Interactions. Holden-Day, San Francisco (1964)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sembira-Nahum, Y., Apelblat, A. & Manzurola, E. Volumetric Properties of Aqueous Solutions of L-Glutamic Acid and Magnesium-L-Glutamate. J Solution Chem 37, 391–401 (2008). https://doi.org/10.1007/s10953-007-9245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-007-9245-z