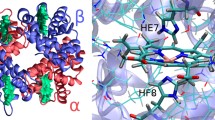
Abstract
Hemoglobin (Hb) is the key metalloprotein within red blood cells involved in oxygen transportation from lungs to body cells. The heme-iron atom inherent within Hb effectuates the mechanism of oxygen transportation and carbon dioxide removal. Structural investigations on avian Hb are limited when compared with the enormous work has been carried out on mammalian Hb. Here, the crystal structure of T-state methemoglobin (T-metHb) from domestic duck (Anas platyrhynchos), a low oxygen affinity avian species, determined to 2.1Å resolution is presented. Duck T-metHb crystallized in the orthorhombic space group C2221 with unit cell parameters a = 59.89, b = 109.42 and c = 92.07Å. The final refined model with R-factor: 19.5% and Rfree: 25.2% was obtained. The structural analysis reveals that duck T-metHb adopts a unique quaternary structure that is distinct from any of the avian liganded Hb structures. Moreover, it closely resembles the deoxy Hb of bar-headed goose, a high oxygen-affinity species. Besides the amino acid αPro119 located in the α1β1 interface, a unique quaternary structure with a constrained heme environment is attributed for the intrinsic low oxygen-affinity of duck Hb. This study reports the first protein crystal structure of low oxygen-affinity avian T-metHb from Anas platyrhynchos.






Similar content being viewed by others
References
Monod J, Wyman J, Changeux JP (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118. https://doi.org/10.1016/S0022-2836(65)80285-6
Perutz MF (1970) Stereochemistry of cooperative effects in haemoglobin: haem–haem interaction and the problem of allostery. Nature 228:726–734
Perutz MF (1972) Nature of haem–haem interaction. Nature 237:495–499
Baldwin J, Chothia C (1979) Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol 129:175–220
Perutz MF (1989) Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys 22:139–237
Perutz MF, Fermi G, Luisi B et al (1987) Stereochemistry of cooperative mechanisms in hemoglobin. Acc Chem Res 20:309–321
Lenfant C, Kooyman GL, Elsner R, Drabek CM (1969) Respiratory function of blood of the Adélie penguin Pygoscelis adeliae. Am J Physiology-Legacy Content 216:1598–1600
Bartlett GR (1970) Patterns of phosphate compounds in red blood cells of man and animals. In: Red cell metabolism and function: Proceedings of the first international conference on red cell metabolism and function, held at the University of Michigan, Ann Arbor, October 1–3, 1969. Springer, pp 245–256
Rollema HS, Bauer C (1979) The interaction of inositol pentaphosphate with the hemoglobins of highland and lowland geese. J Biol Chem 254:12038–12043
Weber RE (1995) Hemoglobin adaptations to hypoxia and altitude-the phylogenetic perspective. Hypoxia Brain 31–44
Knapp JE, Oliveira MA, **e Q et al (1999) The structural and functional analysis of the hemoglobin D component from chicken. J Biol Chem 274:6411–6420
Swan LW (1970) Goose of the Himalayas. Nat Hist 79:691
Liang Y-H, Liu X-Z, Liu S-H, Lu G-Y (2001) The structure of greylag goose oxy haemoglobin: the roles of four mutations compared with bar-headed goose haemoglobin. Acta Crystallogr D Biol Crystallogr 57:1850–1856
Liang Y, Hua Z, Liang X et al (2001) The crystal structure of bar-headed goose hemoglobin in deoxy form: the allosteric mechanism of a hemoglobin species with high oxygen affinity. J Mol Biol 313:123–137
Liu X-Z, Li S-L, **g H et al (2001) Avian haemoglobins and structural basis of high affinity for oxygen: structure of bar-headed goose aquomet haemoglobin. Acta Crystallogr D Biol Crystallogr 57:775–783
Zhang J, Hua Z, Tame JRH et al (1996) The crystal structure of a high oxygen affinity species of haemoglobin (bar-headed goose haemoglobin in the oxy form). J Mol Biol 255:484–493
Black CP, Tenney SM (1980) Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir Physiol 39:217–239
Perutz MF (1983) Species adaptation in a protein molecule. Mol Biol Evol 1:1–28
Shaanan B (1983) Structure of human oxyhaemoglobin at 2· 1resolution. J Mol Biol 171:31–59
Holle JP, Meyer M, Scheid P (1977) Oxygen affinity of duck blood determined by in vivo and in vitro technique. Respir Physiol 29:355–361
Petschow D, Wurdinger I, Baumann R et al (1977) Causes of high blood O2 affinity of animals living at high altitude. J Appl Physiol 42:139–143
Neelagandan K, Moorthy PS, Balasubramanian M, Ponnuswamy MN (2007) Crystallization of sheep (Ovis aries) and goat (Capra hircus) haemoglobins under unbuffered low-salt conditions. Acta Crystallogr Sect F Struct Biol Cryst Commun 63:887–889
Kamariah N, Ponnuraj SM, Moovarkumudalvan B, Ponnuswamy MNG (2014) Structural studies on a low oxygen affinity hemoglobin from mammalian species: Sheep (Ovis aries). Biochem Biophys Res Commun 450:36–41
Moorthy PS, Neelagandan K, Balasubramanian M, Ponnuswamy MNG (2009) Purification, crystallization and preliminary X-Ray Diffraction studies on Goat (Capra hircus) Hemoglobin-A low Oxygen Affinity species. Protein Pept Lett 16:454–456
Sathya Moorthy P, Neelagandan K, Balasubramanian M, Ponnuswamy MN (2009) Purification, crystallization and preliminary X-ray diffraction studies on avian haemoglobin from pigeon (Columba livia). Acta Crystallogr Sect F Struct Biol Cryst Commun 65:120–122
Balasubramanian M, Sathya Moorthy P, Neelagandan K, Ponnuswamy MN (2009) Purification, crystallization and preliminary crystallographic study of haemoglobin from camel (Camelus dromedarius): a high oxygen-affinity lowland species. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:773–775
Balasubramanian M, Moorthy PS, Neelagandan K, Gounder Ponnuswamy MN (2009) Preliminary crystallographic study of Hemoglobin from Buffalo (Bubalus bubalis): a low Oxygen Affinity species. Protein Pept Lett 16:213–215
Balasubramanian M, Sathya Moorthy P, Neelagandan K et al (2014) Structure of liganded T-state haemoglobin from cat (Felis silvestris Catus), a low oxygen-affinity species, in two different crystal forms. Acta Crystallogr D Biol Crystallogr 70:1898–1906
Balasubramanian M, Moorthy PS, Neelagandan K, Ponnuswamy MN (2009) Purification, crystallization and preliminary crystallographic study of low oxygen-affinity haemoglobin from cat (Felis silvestris Catus) in two different crystal forms. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:313–316
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bartels KS, Klein C (2003) The automar Manual, v. 1.4. MAR Research GmbH, Norderstedt, Germany
McCoy AJ, Grosse-Kunstleve RW, Adams PD et al (2007) Phaser crystallographic software. J Appl Crystallogr 40:658–674
CCP C (1994) Collaborative computational project number 4. Acta Crystallogr D Biol Crystallogr 50:760–763
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Matthews BW (1968) Solvent content of protein crystals. J Mol Biol 33:491–497
Dall’Acqua W, Simon AL, Mulkerrin MG, Carter P (1998) Contribution of domain interface residues to the stability of antibody CH3 domain homodimers. Biochemistry 37:9266–9273
Fermi G, Perutz MF, Shaanan B, Fourme R (1984) The crystal structure of human deoxyhaemoglobin at 1.74 Å resolution. J Mol Biol 175:159–174
Borgese TA, Nagel RL (1977) Differential effects of 2, 3-DPG, ATP and inositol pentaphosphate (IP5) on the oxygen equilibria of duck embryonic, fetal and adult hemoglobins. Comp Biochem Physiol Physiol 56:539–543
Isaacks RE, Harkness DR (1980) Erythrocyte organic phosphates and hemoglobin function in birds, reptiles, and fishes. Am Zool 20:115–129
Arnone A (1972) X-ray diffraction study of binding of 2, 3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237:146–149
Natarajan C, Jendroszek A, Kumar A et al (2018) Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet 14:e1007331
Jaimohan SM, Naresh MD, Mandal AB (2021) Parakeet hemoglobin-its Crystal structure and Oxygen Affinity in Relation to some avian hemoglobins. Protein Pept Lett 28:18–30
Ramesh P, Sundaresan SS, Shobana N et al (2021) Structural studies of hemoglobin from two flightless birds, ostrich and Turkey: insights into their differing oxygen-binding properties. Acta Crystallogr D Struct Biol 77:690–702
Jessen T-H, Weber RE, Fermi G et al (1991) Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanism by protein engineering. Proceedings of the National Academy of Sciences 88:6519–6522
Weber RE, Jessen T-H, Malte H, Tame J (1993) Mutant hemoglobins (alpha 119-Ala and beta 55-Ser): functions related to high-altitude respiration in geese. J Appl Physiol 75:2646–2655
Ganapathy J, Palayam M, Pennathur G et al (2018) Crystal Structure Analysis of Great Cormorant (Phalacrocorax carbo) Hemoglobin to understand its high Oxygen Affinity characteristics by Special Structural features. Protein Pept Lett 25:748–756
Liddington R, Derewenda Z, Dodson E et al (1992) High resolution crystal structures and comparisons of T-state deoxyhaemoglobin and two liganded T-state haemoglobins: T (α-oxy) haemoglobin and T (met) haemoglobin. J Mol Biol 228:551–579
Acknowledgements
We thank Dr M. D. Naresh and Mr S. M. Jaimohan of CLRI, Chennai for their support during data collection. K.N would like to thank UGC, New Delhi, P.S.M and M.B thanks CSIR, Government of India, for the award of Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
P.S.M , K.N. and M.B. contributed for Conceptualization, Methodology, Investigation and Writing original draft. R.R. and M.N.P. contributed for Writing & Editing original draft. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponnuraj, S.M., Kamariah, N., Moovarkumudalvan, B. et al. Molecular Insights of an Avian Species with Low Oxygen Affinity, the Crystal Structure of Duck T-State Methemoglobin. Protein J (2024). https://doi.org/10.1007/s10930-024-10206-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10930-024-10206-z