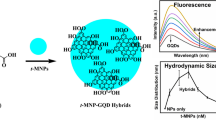
Abstract
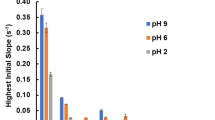
The binding efficiency of graphene oxide and selected transition metal sulfide nanoparticles (Co3S4, NiS, CuS, and ZnS) observed by fluorescence spectroscopy, and corresponding changes observed in the hydrodynamic size by DLS are related to each other. We observed that changes in the size of nanoparticles before and after binding have a similar pattern to the estimated binding constants, which shows a relationship between fluorescence spectroscopy and dynamic light scattering. This relation is yet unknown in the literature to the best of our understanding. Further, the binding mechanism is dependent on the nature of nanoparticles, where ZnS nanoparticles have highest binding towards graphene oxide, followed by CuS, Co3S4, and NiS nanoparticles respectively. The electronic interaction of the aromatic double bonds of GO with the nanoparticles is revealed along with both static and dynamic binding mechanisms. The stability of nanocomposites is also investigated for these titration experiments of nanoparticles and graphene oxide.
Graphical Abstract









Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
M.H. Ayoub et al., Understanding non-covalent interactions of graphene oxide toward transition metal surfaces and relation of binding constants with titration end points from dynamic light scattering studies. J. Appl. Phys. 133(2), 025303 (2023)
Z.U. Hassan et al., Dynamic light scattering and zeta-potential as a tool for understanding the mechanism of pesticides binding toward individual components of transition metal nanoparticles and graphene oxide hybrids. J. Environ. Sci. Health Part B, 2022: p. 1–16
S. Ahmad et al., Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf., a 647, 129057 (2022)
Z. Abbasi et al., Binding efficiency of functional groups towards noble metal surfaces using graphene oxide – metal nanoparticle hybrids. Colloids Surf., a 611, 125858 (2021)
M. Jawad et al., Effect of gold nanoparticles on transmittance and conductance of graphene oxide thin films and efficiency of perovskite solar cells. Appl. Nanosci. 10(2), 485–497 (2019)
J. Prakash et al., Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 154, 62–71 (2020)
J. Liu, L. Cui, D. Losic, Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 9(12), 9243–9257 (2013)
L. Feng, L. Wu, X. Qu, New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 25(2), 168–186 (2013)
R. Yadav et al., Graphene and graphene oxide for fuel cell technology. Ind. Eng. Chem. Res. 57(29), 9333–9350 (2018)
K. Toda, R. Furue, S. Hayami, Recent progress in applications of graphene oxide for gas sensing: a review. Anal. Chim. Acta. 878, 43–53 (2015)
J.-L. Li et al., A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials. 34(37), 9519–9534 (2013)
P. Zheng, N. Wu, Fluorescence and sensing applications of graphene oxide and graphene quantum dots: a review. Chemistry–An Asian Journal. 12(18), 2343–2353 (2017)
M. Mohsin et al., An Insight into the Coating Behavior of Bimetallic Silver and Gold Core-Shell Nanoparticles Plasmonics, 2020
A.J. Shaikh et al., Plasmonic effects, size and biological activity relationship of Au-Ag alloy nanoparticles. J. Nano Res. 54, 98–111 (2018)
M. Jawad et al., Plasmonic effects and size relation of gold-platinum alloy nanoparticles. Adv. Nano Res. 7(3), 169 (2019)
S. Dowland et al., Direct growth of Metal Sulfide Nanoparticle Networks in Solid-State Polymer films for Hybrid Inorganic–Organic Solar cells. Adv. Mater. 23(24), 2739–2744 (2011)
H. Hao, X. Lang, Metal sulfide photocatalysis: visible-light‐induced organic transformations. ChemCatChem. 11(5), 1378–1393 (2019)
Z.-B. Zhai et al., Metal–organic framework derived small sized metal sulfide nanoparticles anchored on N-doped carbon plates for high-capacity energy storage. Dalton Trans. 48(14), 4712–4718 (2019)
O.O. Otelaja et al., Highly conductive Cu2–x S nanoparticle films through room-temperature processing and an order of magnitude enhancement of conductivity via electrophoretic deposition. ACS Appl. Mater. Interfaces. 6(21), 18911–18920 (2014)
S. Goel, F. Chen, W. Cai, Synthesis and biomedical applications of copper sulfide nanoparticles: from sensors to theranostics. Small. 10(4), 631–645 (2014)
A. Kuc, N. Zibouche, T. Heine, Influence of quantum confinement on the electronic structure of the transition metal sulfide T S 2. Phys. Rev. B 83(24), 245213 (2011)
L. **g et al., Nanoparticles weaponized with built-in functions for imaging‐guided cancer therapy. View. 1(2), e19 (2020)
A. Saleem et al., Isoxazole Derivatives against Carbonic Anhydrase: Synthesis, Molecular Docking, MD Simulations, and Free Energy Calculations Coupled with In Vitro Studies ACS Omega, 2022. 7(34): p. 30359–30368
A.J. Shaikh, Exploring the direction of charge transfer in porphyrin – PbSe Quantum dot hybrids. ChemistrySelect. 1(8), 1678–1686 (2016)
A.J. Shaikh, N. Aman, M.A. Yameen, A new methodology for simultaneous comparison and optimization between nanoparticles and their drug conjugates against various multidrug-resistant bacterial strains. Asian Biomed. 13(4), 149–162 (2019)
A.J. Shaikh et al., Binding strength of porphyrin – gold nanoparticle hybrids based on number and type of Linker Moieties and a simple method to calculate inner Filter effects of Gold nanoparticles using fluorescence spectroscopy. J. Phys. Chem. A 119(7), 1108–1116 (2015)
W. Saeed et al., Interactive behavior of graphene quantum dots towards noble metal surfaces. Phys. E: Low-dimensional Syst. Nanostruct. 147, 115596 (2023)
Z. Abbas et al., A simplistic approach to evaluate the power conversion efficiencies for hybrid charge transport layers in open-air fabricated perovskite solar cells. J. Mater. Res. 37(7), 1323–1340 (2022)
F. Agada et al., A step forward toward quantum dots based perovskite solar cells in an ambient environment. Opt. Mater. 129, 112538 (2022)
K. Khan et al., In situ formation of copper nanoparticles in a p(NIPAM-VAA-AAm) terpolymer microgel that retains the swelling behavior of microgels. J. Polym. Eng. 36(3), 287–292 (2016)
S. Ratha et al., Supercapacitors based on patronite–reduced graphene oxide hybrids: experimental and theoretical insights. J. Mater. Chem. A 3(37), 18874–18881 (2015)
C. **e et al., Biomimetic mineralized hierarchical graphene oxide/chitosan scaffolds with adsorbability for immobilization of nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces. 8(3), 1707–1717 (2016)
Q. Wang et al., Graphene oxide directed one-step synthesis of flowerlike graphene@ HKUST-1 for enzyme-free detection of hydrogen peroxide in biological samples. ACS Appl. Mater. Interfaces. 8(47), 32477–32487 (2016)
J. Bai, X. Jiang, A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 85(17), 8095–8101 (2013)
F. Chen et al., A general method for the synthesis of graphene oxide-metal sulfide composites with improved photocatalytic activities. Dyes Pigm. 125, 142–150 (2016)
K.-J. Huang et al., Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. Int. J. Hydrog. Energy. 40(32), 10158–10167 (2015)
H.-C. Tao et al., One-pot facile synthesis of CuS/graphene composite as anode materials for lithium ion batteries. J. Phys. Chem. Solids. 75(11), 1205–1209 (2014)
H. Li et al., Electrochemical immunosensor with N-doped graphene-modified electrode for label-free detection of the breast cancer biomarker CA 15 – 3. Biosens. Bioelectron. 43, 25–29 (2013)
J. Amani, A. Khoshroo, M. Rahimi-Nasrabadi, Electrochemical Immunosensor for the breast cancer marker CA 15–3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim. Acta. 185(1), 1–9 (2018)
K. Krishnamoorthy, G.K. Veerasubramani, S.J. Kim, Hydrothermal synthesis, characterization and electrochemical properties of cobalt sulfide nanoparticles. Mater. Sci. Semiconduct. Process. 40, 781–786 (2015)
A. Molla, M. Sahu, S. Hussain, Synthesis of tunable band gap semiconductor nickel sulphide nanoparticles: Rapid and round the clock degradation of organic dyes. Sci. Rep. 6(1), 1–11 (2016)
S. Riyaz, A. Parveen, A. Azam, Microstructural and optical properties of CuS nanoparticles prepared by sol–gel route. Perspect. Sci. 8, 632–635 (2016)
P. Iranmanesh, S. Saeednia, M. Nourzpoor, Characterization of ZnS nanoparticles synthesized by co-precipitation method. Chin. Phys. B 24(4), 046104 (2015)
P. Huang et al., Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 1, 240 (2011)
H.-C. Hsu et al., Graphene oxide as a promising photocatalyst for CO 2 to methanol conversion. Nanoscale. 5(1), 262–268 (2013)
W. Saeed et al., An insight into the binding behavior of graphene oxide and noble metal nanoparticles. J. Appl. Phys. 129(12), 125302 (2021)
J.R. Lakowicz, Protein fluorescence, Principles of Fluorescence Spectroscopy. 1983, Springer. 341–381
M.V. Manilo, N.I. Lebovka, S. Barany, Effects of sort and concentration of salts on the electrosurface properties of aqueous suspensions containing hydrophobic and hydrophilic particles: validity of the Hofmeister series. J. Mol. Liq. 276, 875–884 (2019)
S. Bhattacharjee, DLS and zeta potential–what they are and what they are not? J. Controlled Release. 235, 337–351 (2016)
Acknowledgements
The research described in this paper was partially financially supported by the Higher Education Commission of Pakistan under National Research Program for Universities with reference no. 20-3369/R&D/HEC/14/978, which was awarded to Dr. A. J. Shaikh.
Author information
Authors and Affiliations
Contributions
S.A. did the benchwork, characterization, and initial draft preparation, M.H.A. and S.A. performed bench work and characterization. F.A. performed the SEM analysis. Z.H., A.W., and M.Y. performed the characterizations. U.F. did the supervision. A.J.S. was involved in conceptualization, visualization, methodology, investigation, supervision, writing, reviewing, editing, and validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, S., Ayoub, M.H., Ahmad, F. et al. Relation between Fluorescence Spectroscopy and Dynamic Light Scattering Revealed by Interaction of Transition Metal Sulfide Nanoparticles and Graphene Oxide. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03002-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03002-w