Abstract
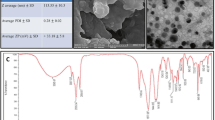
The parthenolide (PLT), a feverfew plant anticancer bioactive compound was coupled with bovine serum albumin (BSA) and coated with chitosan polymers as an appropriate biocompatible drug delivery system to evaluate its anticancer potentials on human HT-29 cancer cell line. The PLT/BSA-chitosan nanoparticles (PBC-NP) were synthesized utilizing a long-term stirring-based assembling technique. The PBC-NPs were characterized by DLS, FTIR, zeta potential, and FESEM analysis. Moreover, the PBC-NP’s cytotoxicity, anti-angiogenic, and anti-metastatic impacts were studied on the HT-29 cell line. Also, Chick chorioallantoic membrane (CAM) and scratch assays were applied to verify the anti-angiogenic and anti-metastatic potential of PBC-NP. Finally, the anti-oxidant activity of PBC-NP was measured by performing ABTS and DPPH assays. The PBC-NP (94.1 nm) decreased the HT-29 cells’survival and down-regulated VEGF/VEGF-R and MMP-9/MMP-2 gene expression, which significantly indicated their anti-angiogenic and anti-metastatic activity, respectively. Moreover, their anti-angiogenic activity was verified by detecting a significant decrease in the count and length of CAM blood vessels. Also, the decreased migration rate of HT-29 cells over the scratched line approves the PBC-NP’s anti-metastatic activity. Finally, the decreased absorbance of free ABTS and DPPH radicals following the increased PBC-NP treatment doses indicated its radical scavenging potential. The PBC-NP was successfully produced as a safe natural antioxidant and biocompatible parthenolide drug delivery system, which notably induced cellular death and decreased angiogenesis and metastasis in human colon cancer cells. Moreover, the BSA- parthenolide interaction can improve the PBC-NP efficiency by providing an intra-particle second delivery system, which will be activated upon degrading the chitosan coating shield of nanoparticles. Therefore, the PBC-NP has the potential to open a novel promising horizon in efficiently treating human colon cancer.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available upon reasonable request from the authors.
References
S. Chakraborti, B.K. Ray, S. Roychoudhury, Handbook of Oxidative Stress in Cancer: Mechanistic Aspects (Springer, 2020)
C. Navarro, Á Ortega, R. Santeliz, B. Garrido, M. Chacín, N. Galban et al., Metabolic reprogramming in Cancer cells: emerging Molecular Mechanisms and Novel Therapeutic Approaches. Pharmaceutics 14(6), 1303 (2022)
P.L. de Sá Junior, D.A.D. Câmara, A.S. Porcacchia, P.M.M. Fonseca, S.D. Jorge, R.P. Araldi et al., The Roles of ROS in Cancer Heterogeneity and Therapy. Oxidative Med. Cell. Longev 2017, 2467940 (2017)
D. Dakowicz, M. Zajkowska, B. Mroczko, Relationship between VEGF family members, their receptors and cell death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci 23(6), 3375 (2022)
H. Li, Z. Qiu, F. Li, C. Wang, The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 14(5), 5865–5870 (2017)
S. Quintero-Fabián, R. Arreola, E. Becerril-Villanueva, J.C. Torres-Romero, V. Arana-Argáez, J. Lara-Riegos et al., Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol 9, 1370 (2019)
J.M. Macharia, R.W. Mwangi, N. Rozmann, K. Zsolt, T. Varjas, P.O. Uchechukwu et al., Medicinal plants with anti-colorectal cancer bioactive compounds: potential game-changers in colorectal cancer management. Biomed. Pharmacother 153, 113383 (2022)
S.D. Steichen, M. Caldorera-Moore, N.A. Peppas, A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci 48(3), 416–427 (2013)
R.R. Sawant, A.M. Jhaveri, A. Koshkaryev, L. Zhu, F. Qureshi, V.P. Torchilin, Targeted transferrin-modified polymeric micelles: enhanced efficacy in vitro and in vivo in ovarian carcinoma. Mol. Pharm 11(2), 375–381 (2014)
S.M.R. Seyedi, A. Asoodeh, M. Darroudi, The human immune cell simulated anti-breast cancer nanorobot: the efficient, traceable, and dirigible anticancer bio-bot. Cancer Nanotechnol 13(1), 1–24 (2022)
F.A. Oskooei, J. Mehrzad, A. Asoodeh, A. Motavalizadehkakhky, Multi-spectroscopic characteristics of olive oil-based Quercetin nanoemulsion (QuNE) interactions with calf thymus DNA and its anticancer activity. J. Mol. Liq 367, 120317 (2022)
F. Ahmadi Oskooei, J. Mehrzad, A. Asoodeh, A. Motavalizadehkakhky, Olive oil-based quercetin nanoemulsion (QuNE)’s interactions with human serum proteins (HSA and HTF) and its anticancer activity. Journal of Biomolecular Structure and Dynamics. 2021:1–14
A.P. Retnakumari, C.D. Nandan, J. Somaraj, J. Antony, V.V. Alex, B.S. Vinod et al., Chitosan Encapsulation enhances the bioavailability and tissue Retention of Curcumin and improves its efficacy in preventing B [a] P-induced lung carcinogenesis
F. Pourianezhad, S. Tahmasebi, S. Nikfar, M. Mirhoseini, V. Abdusi, Review on feverfew, a valuable medicinal plant. Journal of HerbMed Pharmacology. 2016;5
L.D.C. Mannelli, B. Tenci, M. Zanardelli, A. Maidecchi, A. Lugli, L. Mattoli et al., Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 22(7–8), 752–758 (2015)
M. Sztiller-Sikorska, M. Czyz, Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 13(8), 194 (2020)
N.R. Penthala, V. Janganati, T.L. Alpe, S.M. Apana, M.S. Berridge, P.A. Crooks et al., N-[11CH3] Dimethylaminoparthenolide (DMAPT) uptake into orthotopic 9LSF glioblastoma tumors in the rat. Bioorg. Med. Chem. Lett 26(24), 5883–5886 (2016)
A.M. Al-Rahim, R. AlChalabi, A.Z. Al-Saffar, G.M. Sulaiman, S. Albukhaty, T. Belali et al., Folate-methotrexate loaded bovine serum albumin nanoparticles preparation: an in vitro drug targeting cytokines overwhelming expressed immune cells from rheumatoid arthritis patients. Animal Biotechnology. 2021:1–17
S. Al-Musawi, S. Albukhaty, H. Al-Karagoly, G.M. Sulaiman, M.S. Alwahibi, Y.H. Dewir et al., Antibacterial activity of honey/chitosan nanofibers loaded with capsaicin and gold nanoparticles for wound dressing. Molecules 25(20), 4770 (2020)
C. Weber, C. Coester, J. Kreuter, K. Langer, Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm 194(1), 91–102 (2000)
H.H. Nguyen, J. Ryu, J.-H. Che, T.S. Kang, J.K. Lee, C.W. Song et al., Robust size control of bovine serum albumin (BSA) nanoparticles by intermittent addition of a desolvating agent and the particle formation mechanism. Food Chem 141(2), 695–701 (2013)
G.-A. Junter, P. Thébault, L. Lebrun, Polysaccharide-based antibiofilm surfaces. Acta Biomater 30, 13–25 (2016)
M. Mashreghi, M. Faal Maleki, M. Karimi, F. Kalalinia, A. Badiee, M.R. Jaafari, Improving anti-tumour efficacy of PEGylated liposomal doxorubicin by dual targeting of tumour cells and tumour endothelial cells using anti-p32 CGKRK peptide. J. Drug Target 29(6), 617–630 (2021)
N. Jafari, S. Nazeri, Z. Rabiei, S. Tahmasebi Enferadi, R. Behroozi, Growth inhibitory effect of Anthemis haussknechtii root extract, as a source of Parthenolide, on breast cancer cell line. J. Med. plants By-product 5(2), 205–210 (2016)
J. Stetefeld, S.A. McKenna, T.R. Patel, Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys. Rev 8(4), 409–427 (2016)
B. Salopek, D. Krasic, S. Filipovic, Measurement and application of zeta-potential. Rudarsko-geolosko-naftni zbornik 4(1), 147 (1992)
P. Ramasamy, N. Subhapradha, V. Shanmugam, A. Shanmugam, Extraction, characterization and antioxidant property of chitosan from cuttlebone Sepia kobiensis (Hoyle 1885). Int. J. Biol. Macromol 64, 202–212 (2014)
A.M. Abdelgawad, M.E. El-Naggar, S.M. Hudson, O.J. Rojas, Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int. J. Biol. Macromol 94, 96–105 (2017)
K. Abrosimova, O. Shulenina, S. Paston, editors. FTIR study of secondary structure of bovine serum albumin and ovalbumin. Journal of physics: conference series; 2016: IOP Publishing
M. Abbas, T. Hussain, M. Arshad, A.R. Ansari, A. Irshad, J. Nisar et al., Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol 140, 871–876 (2019)
C. Qin, H. Li, Q. **ao, Y. Liu, J. Zhu, Y. Du, Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym 63(3), 367–374 (2006)
M.J. Ansari, D. Bokov, A. Markov, A.T. Jalil, M.N. Shalaby, W. Suksatan et al., Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell. Communication and Signaling 20(1), 1–23 (2022)
Y. Fontebasso, S.M. Dubinett, Drug development for metastasis prevention. Crit. Reviews™ Oncog. 20, 5–6 (2015)
D. Weinstat-Saslow, P.S. Steeg, Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J 8(6), 401–407 (1994)
M.T. Islam, A.B. Khalipha, R. Bagchi, M. Mondal, S.Z. Smrity, S.J. Uddin et al., Anticancer activity of Thymol: a literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB life 71(1), 9–19 (2019)
Y. Zhang, M. Hu, L. Liu, X.-L. Cheng, J. Cai, J. Zhou et al., Anticancer effects of rosmarinic acid in OVCAR-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of lncRNA MALAT-1 expression. J. BUON 23(3), 763–768 (2018)
M.A. Tomeh, R. Hadianamrei, X. Zhao, A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci 20(5), 1033 (2019)
A. Rauf, M. Imran, I.A. Khan, M. ur-Rehman, S.A. Gilani, Z. Mehmood et al., Anticancer potential of quercetin: a comprehensive review. Phytother. Res 32(11), 2109–2130 (2018)
H.E. Mahood, M.K. Abbas, N.A. Zahid, Micropropagation of Feverfew (Tanacetum parthenium) and quantification of Parthenolide Content in its Micropropagated and conventionally grown plants. Horticulturae 8(1), 50 (2022)
T. Gunasekaran, T. Haile, T. Nigusse, M.D. Dhanaraju, Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 4, S1–S7 (2014)
X. Zhou, J. Lu, J. Jiang, X. Li, M. Lu, G. Yuan et al., Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity. Nanoscale Res. Lett 9(1), 1–7 (2014)
J. Agnes, M.S. Muthu, P. Ajith, M. Selvakumar, M. Presheth, D.P. Anand, Preparation and characterization of Chitosan-Encapsulated Cobalt Oxide Nanoparticles modified with folic acid. Journal of Inorganic and Organometallic Polymers and Materials. 2022:1–7
T.H. Abdtawfeeq, Z.A. Farhan, K. Al-Majdi, M.A. Jawad, R.S. Zabibah, Y. Riadi et al., Ultrasound-assisted and One-Pot synthesis of New Fe3O4/Mo-MOF magnetic Nano Polymer as a strong Antimicrobial Agent and efficient nanocatalyst in the Multicomponent synthesis of Novel pyrano [2, 3-d] pyrimidines derivatives. Journal of Inorganic and Organometallic Polymers and Materials. 2022:1–12
M. Aida, N. Alonizan, M. Hussein, M. Hjiri, O. Abdelaziz, R. Attaf et al., Facile synthesis and antibacterial activity of bioplastic membrane containing in doped ZnO/cellulose acetate nanocomposite. J. Inorg. Organomet. Polym Mater 32(4), 1223–1233 (2022)
P. Gao, G. **a, Z. Bao, C. Feng, X. Cheng, M. Kong et al., Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol 91, 716–723 (2016)
Y. Zhang, R. Dong, Y. Park, M. Bohner, X. Zhang, K. Ting et al., Controlled release of NELL-1 protein from chitosan/hydroxyapatite-modified TCP particles. Int. J. Pharm 511(1), 79–89 (2016)
X. Wan, X. Zheng, X. Pang, Z. Zhang, T. **g, W. Xu et al., The potential use of lapatinib-loaded human serum albumin nanoparticles in the treatment of triple-negative breast cancer. Int. J. Pharm 484(1–2), 16–28 (2015)
P.M. Carron, A. Crowley, D. O’Shea, M. McCann, O. Howe, M. Hunt et al., Targeting the folate receptor: improving efficacy in inorganic medicinal chemistry. Curr. Med. Chem 25(23), 2675–2708 (2018)
A. Karmakar, Y. Xu, T. Mustafa, G. Kannarpady, S. Bratton, A. Radominska-Pandya et al., Nanodelivery of parthenolide using functionalized nanographene enhances its anticancer activity. RSC Adv 5(4), 2411–2420 (2015)
L. Noorani, M. Stenzel, R. Liang, M.H. Pourgholami, D.L. Morris, Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnol. 13(1), 1–12 (2015)
M.P. Baranello, L. Bauer, C.T. Jordan, D.S. Benoit, Micelle delivery of parthenolide to acute myeloid leukemia cells. Cell. Mol. Bioeng 8(3), 455–470 (2015)
X. **, J. Zhou, Z. Zhang, H. Lv, The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis. Artif. cells Nanomed. Biotechnol. 46(sup3), S931–S942 (2018)
X. **, Q. Yang, N. Cai, Z. Zhang, A cocktail of betulinic acid, parthenolide, honokiol and ginsenoside Rh2 in liposome systems for lung cancer treatment. Nanomedicine 15(1), 41–54 (2020)
Acknowledgements
This work was supported by, Islamic Azad University, Tehran, Iran, and thus is appreciated by the author.
Funding
This research was performed at a personal expense in the laboratory of the Islamic Azad University of Tehran.
Author information
Authors and Affiliations
Contributions
Ahmed Ibrahim Albosultan: Methodology, Investigation, Formal analysis, Software, and Writing-Original draft. Maryam Ghobeh and Masoud Homayouni Tabrizi: Supervision, Data curation, Conceptualization, Validation, and Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Declaration of Competing Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albosultan, A.I., Ghobeh, M. & Tabrizi, M.H. The Anticancer, Anti-metastatic, Anti-oxidant, and Anti-angiogenic Activity of Chitosan-coated Parthenolide/Bovine Serum Albumin Nanoparticles. J Inorg Organomet Polym 33, 841–852 (2023). https://doi.org/10.1007/s10904-023-02541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02541-y