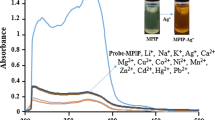
Abstract
Fluorescence and colorimetric sensors have gained significant traction in diverse scientific domains, including environmental, agricultural, and pharmaceutical chemistry. This article comprehensively surveys recent advancements in develo** sensors employing 1,4-dihydroxyanthraquinone(1,4-DHAQ). The study delves into the unique properties of 1,4-dihydroxyanthraquinone(1,4-DHAQ) as a sensor, focusing on its capacity to detect Cu2+ ions and elucidating its fluorescence quenching mechanisms. Furthermore, the interaction of dihydroxyanthraquinone with Ga(III), Al(III), and In(III) ions is explored under both aqueous and non-aqueous conditions, leading to the formation of distinctive fluorescent species. The investigation extends to factors influencing ligand behavior, including time dependency, temperature, solvent type, counterions, and pH levels. These key parameters are systematically analyzed to understand sensor performance better. In conclusion, the article investigates the utility of the 1,4-dihydroxyanthraquinone-Zn2+ probe as a versatile sensing platform for phosphate anions, particularly in live cell imaging. The findings contribute to the evolving landscape of sensor technologies, offering insights into the diverse applications and potential advancements in this burgeoning field.
Graphical Abstract


















Similar content being viewed by others
Availability of Data and Material
All the written material is new not a copy.
Code Availability
Not Applicable.
References
Fouillaud M et al (2016) Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar Drugs 14(4):64
Diaz AN (1990) Absorption and emission spectroscopy and photochemistry of 1, 10-anthraquinone derivatives: a review. J Photochem Photobiol A 53(2):141–167
Gessler N, Egorova A, Belozerskaya T (2013) Fungal anthraquinones. Appl Biochem Microbiol 49:85–99
Noushija MK et al (2022) Selective recognition and reversible “Turn-Off” fluorescence sensing of acetate (CH3COO−) anion at Ppb level using a simple quinizarin fluorescent dye. Chemistry 4(4):1407–1416
Zhang J et al (2016) Dihydroxyanthraquinone derivatives: natural dyes as blue-light-sensitive versatile photoinitiators of photopolymerization. Polym Chem 7(47):7316–7324
Dumur F (2023) Recent advances on Anthraquinone-based photoinitiators of polymerization. Eur Polym J 191:112039
Román Ceba M, Fernández-Gutiérrez A, Mahedero M (1983) Fluorimetrische Bestimmung von Mg (II)-Spuren mit 1, 8-Dikydroxyanthrachinon. Microchim Acta 80:85–94
Das S et al (2015) ESIPT and CHEF based highly sensitive and selective ratiometric sensor for Al 3+ with imaging in human blood cells. New J Chem 39(11):8582–8587
Sinha S et al (2015) Exploring 1, 4-dihydroxyanthraquinone as long-range emissive ratiometric fluorescent probe for signaling Zn2+/PO43−: Ensemble utilization for live cell imaging. J Photochem Photobiol B 148:181–187
Quinti L et al (2003) A study of the luminescent complexes formed by the dye 1, 4-dihydroxyanthraquinone (quinizarin) and Ga (III) and In (III). J Photochem Photobiol A 155(1–3):93–106
Ceba MR, Fernandez-Gutierrez A, Sánchez CM (1985) Some observations on the use of 1, 4-dihydroxyanthraquinone as a fluorometric reagent for traces of lithium. Microchem J 32(3):286–292
Hou L et al (2017) An anthraquinone-based highly selective colorimetric and fluorometric sensor for sequential detection of Cu 2+ and S 2− with intracellular application. J Mater Chem B 5(45):8957–8966
Somma R, Granieri D, Troise C, Terranova C, De Natale G, Pedone M (2017) Modelling of hydrogen sulfide dispersion from the geothermal power plants of Tuscany (Italy). Sci Total Environ 583:408−420
Prodromidis MI, Veltsistas PG, Karayannis MI (2000) Electrochemical study of chemically modified and screen-printed graphite electrodes with [SbVO (CHL) 2] Hex. Application for the selective determination of sulfide. Anal Chem 72(17):3995–4002
Lambert TW et al (2006) Hydrogen sulfide (H2S) and sour gas effects on the eye. A historical perspective. Sci Total Environ 367(1):1–22
Beauchamp R et al (1984) A critical review of the literature on hydrogen sulfide toxicity. CRC Crit Rev Toxicol 13(1):25–97
Wang Y et al (2013) 1, 4-Dihydroxyanthraquinone–Cu 2+ ensemble probe for selective detection of sulfide anion in aqueous solution. Anal Methods 5(20):5493–5500
Lou X et al (2012) An indirect approach for anion detection: the displacement strategy and its application. Chem Commun 48(68):8462–8477
Kaur N (2022) Anthraquinone appended chemosensors for fluorescence monitoring of anions and/or metal ions. Inorg Chim Acta 536:120917
Chae MY, Cherian XM, Czarnik AW (1993) New reagents for the syntheses of fluorescent chemosensors. Anthrylogous ethylene dibromides. J Org Chem 58(21):5797–5801
Bissell RA, De Silva AP, Gunaratne HQN, Lynch PLM, Maguire GEM, Mccoy, CP, Sandanayake, KRAS (1994) ChemInform abstract: fluorescent PET (Photoinduced Electron Transfer) sensors. ChemInform 25
Fukuhara G (2020) Analytical supramolecular chemistry: Colorimetric and fluorimetric chemosensors. J Photochem Photobiol C 42:100340
HeeáLee M, SeungáKim J (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 44(13):4185–4191
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114(8):4564–4601
Godbole G, Pathak S (1986) Chemical transformations of 1-methyl-2α-acetoxy-4β-isopropenyl bicyclo (3, 1, 0) hexane. Curr Sci 55(7):343–345
Kiraly R, Martin RB (1982) Metal ion binding to daunorubicin and quinizarin. Inorg Chim Acta 67:13–18
Roman M et al (1986) Study of the 1, 4-dihydroxyanthraquinone-yttrium (III) complex in solution and in the solid state. Microchem J 34(3):270–276
Coble HD, Holtzclaw HF Jr (1974) Chelate polymers of copper (II) with various dihydroxyquinoid ligands. J Inorg Nucl Chem 36(5):1049–1053
Allen NS et al (1987) Spectroscopic properties of a novel fluorescent complex of aluminium and quinizarin. J Photochem 38:365–373
Hu Y-Z, An J-Y, Jiang L-J (1994) Studies on the chelation of hypocrellin A with aluminium ion and the photodynamic action of the resulting complex. J Photochem Photobiol, B 22(3):219–227
Allen N, Richards A (1990) Spectroscopic, photochemical and photoconductive properties of a novel fluorescent polymeric complex of quinizarin and aluminium. Eur Polymer J 26(11):1229–1235
Gomez-Hens A, Valcarcel M (1982) Spectrofluorimetric determination of inorganic anions: a review. Analyst 107(1274):465–494
Kaur N, Gauri (2022) Anthraquinone appended chemosensors for fluorescence monitoring of anions and/or metal ions. Inorganica Chim Acta 536:120917
Langdon-Jones EE, Pope SJA (2014) The coordination chemistry of substituted anthraquinones: Developments and applications. Coord Chem Rev 269:32–53
Mariappan K, Sykes AG (2013) The chemistry of constrained crown ring systems and fluorescence sensor applications. J Incl Phenom Macrocycl Chem 75:23–30
Quinti L et al (2003) A study of the strongly fluorescent species formed by the interaction of the dye 1, 4-dihydroxyanthraquinone (quinizarin) with Al (III). J Photochem Photobiol A 155(1–3):79–91
Mohanty T, Choudhury SD (2022) Configurable fluorescent constructs for detection and discrimination of fluoride and biological phosphates. J Mol Liq 358:119194
Kar C, Das G (2013) A retrievable fluorescence “TURN ON” sensor for sulfide anions. J Photochem Photobiol A 251:128–133
Chen W et al (2015) Selective, highly sensitive fluorescent probe for the detection of sulfur dioxide derivatives in aqueous and biological environments. Anal Chem 87(1):609–616
Tayade K et al (2014) Architecture of dipodal ratiometric motif showing discrete nanomolar response towards fluoride ion. Sens Actuators B Chem 202:1333–1337
Khairnar N et al (2014) Novel fluorescent chemosensing of CN− anions with nanomolar detection using the Zn 2+–isonicotinohydrazide metal complex. RSC Adv 4(79):41802–41806
Ganjali M et al (2009) A new Tb3+-selective fluorescent sensor based on 2-(5-(dimethylamino) naphthalen-1-ylsulfonyl)-N-henylhydrazinecarbothioamide. Spectrochim Acta Part A Mol Biomol Spectrosc 74(2):575–578
Yadav UN et al (2014) Quinoline-based chemosensor for fluoride and acetate: a combined experimental and DFT study. Sens Actuators B: Chem 197:73–80
Sharma D et al (2014) Anion selective chromogenic and fluorogenic chemosensor and its application in breast cancer live cell imaging. RSC Adv 4(78):41446–41452
Gunnlaugsson T et al (2006) Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord Chem Rev 250(23–24):3094–3117
Tang L et al (2013) Rapid and highly selective relay recognition of Cu (II) and sulfide ions by a simple benzimidazole-based fluorescent sensor in water. Sens Actuators B: Chem 185:188–194
Wu J et al (2022) “Off-on” fluorescence probe based on green emissive carbon dots for the determination of Cu2+ ions and glyphosate and development of a smart sensing film for vegetable packaging. Microchim Acta 189(3):131
Sinha S et al (2014) Triazole-based Zn2+-specific molecular marker for fluorescence bioimaging. Anal Chim Acta 822:60–68
Park GJ et al (2014) A single chemosensor for multiple analytes: fluorogenic detection of Zn 2+ and OAc− ions in aqueous solution, and an application to bioimaging. New J Chem 38(6):2587–2594
Sivaraman G, Anand T, Chellappa D (2014) Pyrene based selective–ratiometric fluorescent sensing of zinc and pyrophosphate ions. Anal Methods 6(7):2343–2348
Kiyose K et al (2006) Development of a ratiometric fluorescent zinc ion probe in near-infrared region, based on tricarbocyanine chromophore. J Am Chem Soc 128(20):6548–6549
Singh N, Jang DO (2011) Tetrapodal receptors for selective fluorescent sensing of AMP: analyte-induced conformational restriction to persuade fluorescence enhancement. Tetrahedron Lett 52(20):2608–2610
Sinha S et al (2015) Highly photostable zinc selective molecular marker bearing flexible pivotal unit: opto-fluorescence enhancement effect and imaging applications in living systems. Dalton Trans 44(20):9506–9515
Cheuk D et al (2015) Investigation into solid and solution properties of quinizarin. CrystEngComm 17(21):3985–3997
Wang Y et al (2012) Colorimetric and fluorescence sensing of Cu2+ in water using 1,8-dihydroxyanthraquinone-β-cyclodextrin complex with the assistance of ammonia. Talanta 94:172–177
Goshisht M, Tripathi N (2021) Fluorescence-based sensors as an emerging tool for anion detection: Mechanism, sensory materials and applications. J Mater Chem C 9:9820–9850
Azevêdo L et al (2007) Evaluation of the Formation and Stability of Hydroxyalkylsulfonic Acids in Wines. J Agric Food Chem 55:8670–8680
Pal A et al (2021) A detailed insight into anion sensing based on intramolecular charge transfer (ICT) mechanism: A comprehensive review of the years 2016 to 2021. Coord Chem Rev 448:214167
Ali R, Razi SS, Gupta RC, Dwivedi SK, Misra A (2016) An efficient ICT based fluorescent turn-on dyad for selective detection of fluoride and carbon dioxide. New J Chem 40:162–170
Liu X et al (2018) Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175(2):502–513.e13
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116:3029–3085
Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 11:173–186
Beer P, Gale P (2001) Anion recognition and sensing: the state of the art and future perspectives. Angew Chem Int Ed Engl 40:486–516
Sharma D et al (2015) Bioimaging application of a novel anion selective chemosensor derived from vitamin B6 cofactor. J Photochem Photobiol B: Biol 148:37–42
Martínez-Máñez R, Sancenón F (2003) Fluorogenic and chromogenic chemosensors and reagents for anions. Chem Rev 103:4419–4476
Bowman-James K, Bianchi A, García-España E (2012) Anion coordination chemistry. John Wiley & Sons
Prasanna de Silva A et al (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Funding
None.
Author information
Authors and Affiliations
Contributions
The basic idea and outline of the present article are the collective contributions of all authors. They all read and approved the final manuscript of this research article. Kanwal Shahzadi and Sadia Asim play a key role in collecting data regarding the Dual-emission fluorescent chemical sensor properties of 1,4 dihydroxy anthraquinones and their applications for detecting various metal ions. Asim Mansha collected cell imagining applications of Anthraquinone derivatives. The first draft of the manuscript was written by Kanwal Shahzadi which Sadia Asim and Asim Mansha later refined.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conset to Participate
Yes, i got permission.
Consent for Publication
Yes, u can publish it.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahzadi, K., Mansha, A. & Asim, S. The Fluorescence Sensing Capability of 1,4-dihydroxyanthraquinone Towards Metal Ions and Imaging Cells. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03625-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03625-9