Abstract
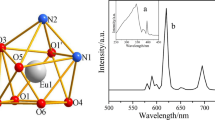
Two novel ternary rare earth complexes of Tb(III) and Dy(III) perchlorates with bis(benzoylmethyl) sulfoxide (L) and benzoic acid (L′) had been synthesized and characterized by elemental analysis, coordination titration analysis, molar conductivity, IR, TG-DSC, 1HNMR and UV spectra. The results indicated that the composition of these complexes was REL5L′(ClO4)2·nH2O (RE= Tb(III), Dy(III); L=C6H5COCH2SOCH2COC6H5, L′=C6H5COO; n = 6,8). The fluorescence spectra illustrated that the ternary rare earth complexes presented stronger fluorescence intensities, longer lifetimes and higher fluorescence quantum efficiencies than the binary rare earth complexes REL5·(ClO4)3·2H2O. After the introduction of the second ligand benzoic acid group, the relative fluorescence emission intensities and fluorescence lifetimes of the ternary complexes REL5L′(ClO4)2·nH2O (RE= Tb(III), Dy(III)) enhanced more obviously than the binary complexes. This indicated that the presence of both organic ligands bis(benzoylmethyl) sulfoxide and the second ligand benzoic acid could sensitize fluorescence intensities of rare earth ions, and the introduction of benzoic acid group was resulted in the enhancement of the fluorescence properties of the ternary rare earth complexes. The phosphorescence spectra were also discussed.








Similar content being viewed by others
References
Kukhta A, Kolesnik E, Grabchev I, Sali S (2006) Spectral and luminescent properties and electroluminescence of polyvinylcarbazole with 1, 8-Naphthalimide in the Side Chain. J Fluoresc 16:375–378
Lehn JM (1990) Perspectives in supramolecular chemistry—from molecular recognition towards molecular information processing and self-organization. Angew Chem Int Ed Engl 29:1304–1319
Wu FB, Han SQ, Zhang C, He YF (2002) Synthesis of a highly fluorescent β-diketonate-europium chelate and its utility in time-resolved fluoroimmunoassay of serum total thyroxine. Anal Chem 74:5882–5889
Saha AK, Kross K, Kloszewski ED, Upson DA, Toner JL, Snow RA, Black CDV, Desai VC (1993) Time-Resolved fluorescence of a new europium chelate complex: demonstration of highly sensitive detection of protein and DNA samples. J Am Chem Soc 115:11032–11033
Shi XY, Li WX, Qin CH, Guo L, Sun XJ, Sun XL, Geng GQ (2008) Synthesis and characterization of quaternary complexes of light rare earth perchlorate with diphenyl sulfoxide, benzoic acid and studies on fluorescence of Eu3+ complex. Chinese J Luminesc 29:772–778
Guo L, Li WX, Shi XY, Sun XJ, Sun XL (2009) Enhanced luminescence of rare-earth Tb (III) by Tm (III) in bis(benzoylmethyl) sulfoxide complexes and intra-molecular energy transfer. J Luminesc 129:639–644
Li WX, Guo L, Chen LJ, Shi XY (2008) Synthesis and fluorescence properties of Lanthanide (III) perchlorate complexes with bis(benzoylmethyl) sulfoxide. J Fluoresc 18:1043–1049
Li WX, Zheng YS, Sun XJ, Shi XY, Chai WJ, Ren T (2009) Synthesis and fluorescence properties of Lanthanide (III) perchlorate complexes with naphthyl-naphthalinesulphonylpropyl sulfoxide. J Fluoresc. doi:10.1007/s10895-009-0544-1
Qian GD, Wang MQ (1998) Synthesis, characterization and fluorescence of Eu3+, Tb3+ complexes with heterocyclic. Chinese J Luminesc 19:60–65
Yuan JB, Li JH, Leung LM, So S, Shi JX, Gong ML (2005) Synthesis and photoluminescence of three Eu (III) ternary complexes with new secondary ligands with different structure. J Chinese Rare Earth Soc 22:600–604
Li YY, Yan T, Wang DM, Du B, Wei Q (2005) The luminescent mechanism and application of rare earth complex. J **an Univer (Sci & Tech) 19:113–119
An BL, Gong ML, Li MX (2005) Synthesis and luminescence of a novel conjugated europium complex with 6-aniline carbonyl 2-pyridine carboxylic acid. J Fluoresc 15:613–617
** P, Gu XH, Chen CF (2007) Synthesis, characterization and luminescent properties of new highly luminescent organic ligand and complexes of trivalent rare earth. Spectrochimica Acta Part A 66:667–671
Greary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122
Deacon GB, Phillips RJ (1980) Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev 33:227–250
Rosenthal MR (1973) The myth of the non-coordinating anion. J Chem Educ 50:331–335
Hathaway BJ, Underhill AE (1961) The infrared spectra of some transition-metal perchlorates. J Chem Soc 65:3091–3096
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Spectroscopic study ofluminescence and intramolecular energy transfer of binary andternary rare earth complexes with aromatic carboxylic acids and1, 10-phenanthroline. Spectro Lett 31:603–608
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Intramolecular energy transfer echanism between ligands internary complexes with aromatic acids and 1, 10-phenanthroline. J Photochem Photobiol A Chem 116:209–214
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–841
Dean CRS, Shepherd TM (1975) Evaluation of the intramolecular energy transfer rate constants in crystalline Eu(hfaa)4ButNH31. J Chem Soc Faraday Trans II 71:146–152
Gerisler HF, Hellwege KH (1953) Spectrum and luminescent mechanism of crystal Tb(BrO3)3. Physik 136:293–295
Gerisler HF, Hellwege KH (1953) Zeitschrift für Physik A Hadrons and Nuclei 136:293–302
Acknowledgements
This work was supported by the financial supports from the National Natural Science Foundations of China Research project (20861005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, WX., Chai, WJ., Sun, XJ. et al. Synthesis and Luminescence Properties of Two Novel Lanthanide (III) Perchlorate Complexes with Bis(benzoylmethyl) Sulfoxide and Benzoic Acid. J Fluoresc 20, 873–880 (2010). https://doi.org/10.1007/s10895-010-0633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0633-1