Abstract
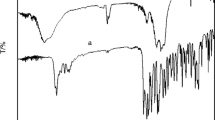
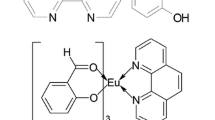
Two novel ternary rare-earth complexes SmL5·L’·(ClO4)2·7H2O and EuL5·L’·(ClO4)2·6H2O (the first ligand L = C6H5COCH2SOCH2COC6H5, the second ligand L’ = C6H4OHCOO−) were synthesized and characterized by element analysis, molar conductivity, coordination titration analysis, IR, TG-DSC, 1HNMR and UV spectra. The detailed luminescence studies on the rare-earth complexes showed that the ternary rare-earth complexes presented stronger fluorescence intensities, longer lifetimes, and higher fluorescence quantum efficiencies than the binary rare-earth materials. After the introduction of the second ligand salicylic acid group, the relative emission intensities and fluorescence lifetimes of the ternary complexes LnL5·L’·(ClO4)2·nH2O (Ln = Sm, Eu; n = 7, 6) enhanced more obviously than the binary complexes LnL5·(ClO4)3·2H2O. This indicated that the presence of both organic ligand bis(benzoylmethyl) sulfoxide and the second ligand salicylic acid could sensitize fluorescence intensities of rare-earth ions, and the introduction of salicylic acid group was a benefit for the fluorescence properties of the ternary rare-earth complexes. The fluorescence spectra, fluorescence lifetime and phosphorescence spectra were also discussed.












Similar content being viewed by others
References
Kukhta A, Kolesnik E, Grabchev I, Sali S (2006) Spectral and luminescent properties and electroluminescence of polyvinylcarbazole with 1, 8-Naphthalimide in the Side Chain. J Fluoresc 16:375–378
Lehn JM (1990) Perspectives in supramolecular chemistry—from molecular recognition towards molecular information processing and self-organization. Angew Chem Int Ed Engl 29:1304–1319
Edward A, Chu TY, Claude C, Sokolik I, Okamoto Y, Dorsinville R (1997) Synthesis and characterization of electroluminescent organo-lanthanide(III) complexes. Synth Met 84:433–434
Kido J, Okamoto Y (2002) Organo lanthanide metal complexes for electroluminescent materials. Chem Rev 102:2357–2368
**n H, Li FY, Shi M, Bian ZQ, Huang CH (2003) Efficient electroluminescence from a new terbium complex. J Am Chem Soc 125:7166–7167
Wu FB, Han SQ, Zhang C, He YF (2002) Synthesis of a highly fluorescent β-diketonate-europium chelate and its utility in time-resolved fluoroimmunoassay of serum total thyroxine. Anal Chem 74:5882–5889
Sabbatini N, Guardigli M, Lehn JM (1993) Luminescent lanthanide complexes as photochemical supramolecular devices. Coord Chem Rev 123:201–228
Niyama E, Brito HF, Cremona M, Teotonio EES, Reyes R, Birto GES, Felinto MCFC (2005) Synthesis and spectroscopic behavior of highly luminescent Eu3+ -dibenzoylmethanate(DBM) complexes with sulfoxide ligands. Spectrochim Acta Part A 61:2643–2649
Shi XY, Li WX, Qin CH, Guo L, Sun XJ, Sun XL, Geng GQ (2008) Synthesis and characterization of quaternary complexes of light rare earth perchlorate with diphenyl sulfoxide, benzoic acid and studies on fluorescence of Eu3+ complex. Chinese J Luminesc 29:772–778
Guo L, Li WX, Shi XY, Sun XJ, Sun XL (2009) Enhanced luminescence of rare-earth Tb (III) by Tm (III) in bis(benzoylmethyl) sulfoxide complexes and intra-molecular energy transfer. J Luminesc 129:639–644
Qian GD, Wang MQ (1998) Synthesis, characterization and fluorescence of Eu3+, Tb3+ complexes with heterocyclic. Chinese J luminesc 19:60–65
Yuan JB, Li JH, Leung LM, So S, Shi JX, Gong ML (2005) Synthesis and photoluminescence of three Eu (III) ternary complexes with new secondary ligands with different structure. J Chinese Rare Earth Soci 22:600–604
Li YY, Yan T, Wang DM, Du B, Wei Q (2005) The luminescent mechanism and application of rare earth complex. J **an Univer (Sci & Tech) 19:113–119
Lin PH, Wang SH, Chang SL (2007) Synthesis and characterization of do** terbrium-dichlorosalicylic acid-N-phenyl-β-naphthylamine complexes. Spectroscopy and Spectral Analysis 27:123–125
Zhang Z, Pan WL (2007) Study on synthesis of the complex Eu(Sal)2(phen)2(NO3) and its fluorescence performance. Fine and Specialty Chemicals 15:15–17
Greary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122
Deacon GB, Phillips RJ (1980) Relationships between the carbon–oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev 33:227–250
Sun WJ, Yang XW, Zhang GH, Zhu L, Gao SL (2006) Spectra of rare earth complexes with salicylate. Acta Photonica Sinica 35:1593–1596
Rosenthal MR (1973) The myth of the non-coordinating anion. J Chem Educ 50:331–335
Hathaway BJ, Underhill AE (1961) The infrared spectra of some transition-metal perchlorates. J Chem Soc 65:3091–3096
Shang HR, Zhao XS (1997) The laser-inducefluorescence of Eu3+, Tb3+ conjugate complex. J Phys 13:586–592
Qiang S (1993) Chemistry of rare earths. Henan Technology &Science Press, Zhengzhou, pp 304–314 (in Chinese)
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Spectroscopic study ofluminescence and intramolecular energy transfer of binary andternary rare earth complexes with aromatic carboxylic acids and1, 10-phenanthroline. Spectro Lett 31:603–608
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Intramolecular energy transfer echanism between ligands internary complexes with aromatic acids and 1, 10-phenanthroline. J Photochem Photobiol A Chem 116:209–214
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–841
Dean CRS, Shepherd TM (1975) Evaluation of the intramolecular energy transfer rate constants in crystalline Eu(hfaa)4ButNH31. J Chem Soc Faraday Trans II 71:146–152
Zhang RH, Shen PW (1987) The chemistry of rare earth elements. Tian **g Technology & Science Press, Tian **g (in Chinese)
Wang YG, Gong MX (1994) Energy transfer from aromatic monoketones to rare earth ions. Acta Scientiarum Naturalium Universitatis Jilinensis 3:89–94
Acknowledgements
This work was supported by the financial supports from the National Natural Science Foundations of China Research project (20861005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, XJ., Li, WX., Chai, WJ. et al. The Studies of Enhanced Fluorescence in the Two Novel Ternary Rare-Earth Complex Systems. J Fluoresc 20, 453–461 (2010). https://doi.org/10.1007/s10895-009-0567-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-009-0567-7