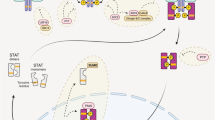
Abstract
Purpose
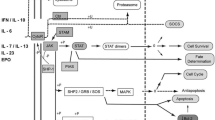
STAT1 gain-of-function (GOF) and dominant-negative (DN) STAT3 syndromes share clinical manifestations including infectious and inflammatory manifestations. Targeted treatment with Janus-kinase (JAK) inhibitors shows promising results in treating STAT1 GOF-associated symptoms while management of DN STAT3 patients has been largely supportive. We here assessed the impact of ruxolitinib on the JAK-STAT1/3 pathway in DN STAT3 patients’ cells.
Methods
Using flow cytometry, immunoblot, qPCR, and ELISA techniques, we examined the levels of basal STAT1 and phosphorylated STAT1 (pSTAT1) of cells obtained from DN STAT3, STAT1 GOF patients, and healthy donors following stimulation with type I/II interferons (IFNs) or interleukin (IL)-6. We also describe the impact of ruxolitinib on cytokine-induced STAT1 signaling in these patients.
Results
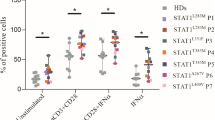
DN STAT3 and STAT1 GOF resulted in a similar phenotype characterized by increased STAT1 and pSTAT1 levels in response to IFNα (CD3+ cells) and IFNγ (CD14+ monocytes). STAT1-downstream gene expression and C-X-C motif chemokine 10 secretion were higher in most DN STAT3 patients upon stimulation compared to healthy controls. Ex vivo treatment with the JAK1/2-inhibitor ruxolitinib reduced cytokine responsiveness and normalized STAT1 phosphorylation in DN STAT3 and STAT1 GOF patient’ cells. In addition, ex vivo treatment was effective in modulating STAT1 downstream signaling in DN STAT3 patients.
Conclusion
In the absence of effective targeted treatment options for AD-HIES at present, modulation of the JAK/STAT1 pathway with JAK inhibitors may be further explored particularly in those AD-HIES patients with autoimmune and/or autoinflammatory manifestations.







Similar content being viewed by others
Data availability
The raw datasets generated and analyzed for this study and supporting the conclusions will be made available by the corresponding author without undue reservation and on reasonable request to any qualified researcher.
References
Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64.
Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36(4):515–28.
Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19.
Zhang Q, Boisson B, Beziat V, Puel A, Casanova JL. Human hyper-IgE syndrome: singular or plural? Mamm Genome. 2018;29(7-8):603–17.
Tsilifis C, Freeman AF, Gennery AR. STAT3 Hyper-IgE syndrome-an update and unanswered questions. J Clin Immunol. 2021;41:864–80.
Asano T KJ, Zhang P, Rapaport F, Spaan AN, Li J, Lei WT, Pelham SJ, Hum D, Chrabieh M, Han J, Guerin A, Joseph Mackie J et al. Human STAT3 variants underlie autosomal dominant hyper-IgE syndrome by negative dominance. J Exp Med. 2021;218(8).
Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in Human Disease. J Clin Immunol. 2015;35(7):615–23.
Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702.
Kane A, Deenick EK, Ma CS, Cook MC, Uzel G, Tangye SG. STAT3 is a central regulator of lymphocyte differentiation and function. Curr Opin Immunol. 2014;28:49–57.
Haddad E. STAT3: too much may be worse than not enough! Blood. 2015;125(4):583–4.
Al-Shaikhly T, Ochs HD. Hyper IgE syndromes: clinical and molecular characteristics. Immunol Cell Biol. 2019;97(4):368–79.
Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29(3-4):123–30.
Mitchell AL, Urban AK, Freeman AF, Hammoud DA. An unusual pattern of premature cervical spine degeneration in STAT3-LOF. J Clin Immunol. 2021;41(3):576–84.
Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127(25):3154–64.
Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208(8):1635–48.
Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39.
Zhang Y, Ma CA, Lawrence MG, Break TJ, O'Connell MP, Lyons JJ, et al. PD-L1 up-regulation restrains Th17 cell differentiation in STAT3 loss- and STAT1 gain-of-function patients. J Exp Med. 2017;214(9):2523–33.
Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol. 2017;139(5):1629–40 e2.
Bloomfield M, Kanderova V, Parackova Z, Vrabcova P, Svaton M, Fronkova E, et al. Utility of ruxolitinib in a child with chronic mucocutaneous candidiasis caused by a novel STAT1 gain-of-function mutation. J Clin Immunol. 2018;38(5):589–601.
Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. 2015;135(2):551–3.
Acker KP, Borlack R, Iuga A, Remotti HE, Soderquist CR, Okada S, et al. Ruxolitinib response in an infant with very-early-onset inflammatory bowel disease and gain-of-function STAT1 mutation. J Pediatr Gastroenterol Nutr. 2020;71(4):e132–e3.
Mossner R, Diering N, Bader O, Forkel S, Overbeck T, Gross U, et al. Ruxolitinib induces interleukin 17 and ameliorates chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation. Clin Infect Dis. 2016;62(7):951–3.
Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol. 2018;142(5):1665–9.
Bergerson JRE, Freeman AF. An update on syndromes with a Hyper-IgE phenotype. Immunol Allergy Clin North Am. 2019;39(1):49–61.
Yanagimachi M, Ohya T, Yokosuka T, Kajiwara R, Tanaka F, Goto H, et al. The potential and limits of hematopoietic stem cell transplantation for the treatment of autosomal dominant hyper-IgE syndrome. J Clin Immunol. 2016;36(5):511–6.
Harrison SC, Tsilifis C, Slatter MA, Nademi Z, Worth A, Veys P, et al. Hematopoietic stem cell transplantation resolves the immune deficit associated with STAT3-dominant-negative hyper-IgE syndrome. J Clin Immunol. 2021;41(5):934–43.
Grimbacher B, Schaffer AA, Holland SM, Davis J, Gallin JI, Malech HL, et al. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet. 1999;65(3):735–44.
Bhattacharya S, Williamson H, Urban AK, Heller T, Freeman AF. Spontaneous gastrointestinal perforations in STAT3-deficient hyper-IgE syndrome. J Clin Immunol. 2020;40(8):1199–203.
Jiao H, Toth B, Erdos M, Fransson I, Rakoczi E, Balogh I, et al. Novel and recurrent STAT3 mutations in hyper-IgE syndrome patients from different ethnic groups. Mol Immunol. 2008;46(1):202–6.
Woellner C, Gertz EM, Schaffer AA, Lagos M, Perro M, Glocker EO, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125(2):424–32 e8.
Zheng J, van de Veerdonk FL, Crossland KL, Smeekens SP, Chan CM, Al Shehri T, et al. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC). Eur J Immunol. 2015;45(10):2834–46.
Bernabei P, Coccia EM, Rigamonti L, Bosticardo M, Forni G, Pestka S, et al. Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J Leukoc Biol. 2001;70(6):950–60.
Zegeye MM, Lindkvist M, Falker K, Kumawat AK, Paramel G, Grenegard M, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal. 2018;16(1):55.
Zimmerman O, Olbrich P, Freeman AF, Rosen LB, Uzel G, Zerbe CS, et al. STAT1 gain-of-function mutations cause high total STAT1 levels with normal dephosphorylation. Front Immunol. 2019;10:1433.
Goel RR, Nakabo S, Dizon BLP, Urban A, Waldman M, Howard L, et al. Lupus-like autoimmunity and increased interferon response in patients with STAT3-deficient hyper-IgE syndrome. J Allergy Clin Immunol. 2021;147(2):746–9 e9.
Zimmerman O, Rosler B, Zerbe CS, Rosen LB, Hsu AP, Uzel G, et al. Risks of ruxolitinib in STAT1 gain-of-function-associated severe fungal disease. Open Forum Infect Dis. 2017;4(4):ofx202.
Moriya K, Suzuki T, Uchida N, Nakano T, Katayama S, Irie M, et al. Ruxolitinib treatment of a patient with steroid-dependent severe autoimmunity due to STAT1 gain-of-function mutation. Int J Hematol. 2020;112(2):258–62.
Shi JG, Chen X, McGee RF, Landman RR, Emm T, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644–54.
Ogama Y, Mineyama T, Yamamoto A, Woo M, Shimada N, Amagasaki T, et al. A randomized dose-escalation study to assess the safety, tolerability, and pharmacokinetics of ruxolitinib (INC424) in healthy Japanese volunteers. Int J Hematol. 2013;97(3):351–9.
Raedler LA. Jakafi (Ruxolitinib): first FDA-approved medication for the treatment of patients with polycythemia vera. Am Health Drug Benefits. 2015;8(Spec Feature):75–9.
Kornblau SM, Womble M, Cade JS, Lemker E, Qiu YH. Comparative analysis of the effects of sample source and test methodology on the assessment of protein expression in acute myelogenous leukemia. Leukemia. 2005;19(9):1550–7.
Carow B, Rottenberg ME. SOCS3, a major regulator of infection and inflammation. Front Immunol. 2014;5:58.
Kayaoglu B, Kasap N, Yilmaz NS, Charbonnier LM, Geckin B, Akcay A, et al. Stepwise reversal of immune dysregulation due to STAT1 gain-of-function mutation following ruxolitinib bridge therapy and transplantation. J Clin Immunol. 2021;41(4):769–79.
Pelham SJ, Lenthall HC, Deenick EK, Tangye SG. Elucidating the effects of disease-causing mutations on STAT3 function in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2016;138(4):1210–3 e5.
Yokokawa T, Misaka T, Kimishima Y, Wada K, Minakawa K, Sugimoto K, et al. Crucial role of hematopoietic JAK2V617F in the development of aortic aneurysms. Haematologica. 2021;106(7):1910–22.
Acknowledgements
The authors would like to thank the patients and their families for participating in this study.
Funding
This work was supported by the Job Research Foundation (NY, United States); Consejería de Salud de la Junta de Andalucía (SA0051/2020 to O.N.); Agencia de Innovación y Desarrollo de Andalucía (PI-0184-2018 to P.O.), Instituto de Salud Carlos III, Madrid, Spain [Sara Borrell, CD20/00124 to P.B.L, Juan Rodés JR18/00042 to P.O, FIS PI19/01471].
Author information
Authors and Affiliations
Contributions
PO and ON contributed to the conception of the work. PBL, PGH, and IV performed all sample processing and experiments. BdF organized sample ship**. CC, HR, BCG, AME, JML, POA, PO, and ON contributed to the diagnostic and inclusion of DN STAT3 patients. MJC contributed as technician of the cytometry core of the Institute of Biomedicine of Seville. JFNU contributed to the western blot performance analysis. All authors (PBL, PGH, IV, BdF, CC, HR, BCG, AME, JML, POA, MJC, JFNU, JDM, KM, OZ, AF, MSL, SMH, ON, and PO) contributed to the analysis or interpretation of the data, manuscript revision, read, and approved the submitted version. PBL wrote the first draft. PO and ON edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of the Hospitales Universitarios Virgen Macarena and Virgen del Rocío (0243-N-19).
Consent to participate
All patients, family members, and healthy volunteers provided written and signed informed consent at each Spanish participating center (Seville, Malaga, Valencia, and Madrid). The authors affirm that human research participants or their legal guardians provided informed consent for participation and publication of their individual details.
Consent for publication
All authors agreed with the submission and publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lobo, P.B., Guisado-Hernández, P., Villaoslada, I. et al. Ex vivo effect of JAK inhibition on JAK-STAT1 pathway hyperactivation in patients with dominant-negative STAT3 mutations. J Clin Immunol 42, 1193–1204 (2022). https://doi.org/10.1007/s10875-022-01273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01273-x