Abstract
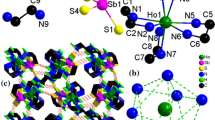
Two rare earth metal thioantimonates [RE(en)4]SbS4·0.5en (RE = Y(1), Tm(2); en = ethylenediamine) were synthesized under mild solvothermal conditions. Compounds 1 and 2 are isostructural, and both crystallize in monoclinic space group P21/n. Crystallographic data for 1: a = 11.0894(19), b = 12.905(2), c = 16.000(3) Å, β = 91.792(4), V = 2288.7(7) Å3, Z = 4. For 2: a = 11.0870(14), b = 12.8977(16), c = 15.986(2) Å, β = 91.879(3), V = 2284.7(5) Å3, Z = 4. The four-en coordinated rare earth complex cation [RE(en)4]3+ formed in situ balances the charge of the [SbS4]3− anion in the crystal structure. The RE3+ ion is in an eight-coordinated environment involving eight N atoms of four en ligands forming a bicapped trigonal prism. Hydrogen bonds link [RE(en)4]3+, [SbS4]3− and en species into a three-dimensional structure. The structure determination of 1 and 2 implies that the ionic radii of rare earth metal ions play an important role on the structures of the rare earth metal thioantimonates.
Index Abstract
Rare earth metal thioantimonates [RE(en)4]SbS4·0.5en (RE = Y, Tm) were synthesized under mild solvothermal conditions, whose structures are related with the ionic radii of the rare earth metal ions.





Similar content being viewed by others
References
Sheldrick WS, Wachhold M (1998) Coord Chem Rev 176:211
**g L, Chen Z, Wang RJ, Proserpio DM (1999) Coord Chem Rev 190–192:707
Sheldrick WS (2000) J Chem Soc Dalton Trans 3041
Dehnen S, Melullis M (2007) Coord Chem Rev 251:1259
Jia DX, Zhang Y, Dai J, Zhu QY, Gu XM (2004) Z Anorg Allg Chem 630:313
Li J, Chen Z, Emge TJ, Yuen T, Proserpio DM (1998) Inorg Chim Acta 273:310
Behrens M, Scherb S, Näther C, Bensch W (2003) Z Anorg Allg Chem 629:1367
Jia DX, Zhang Y, Dai J, Zhu QY, Gu XM (2004) J Solid State Chem 177:2477
Schaefer M, Näther C, Lehnert N, Bensch W (2004) Inorg Chem 43:2914
Kiebach R, Studt F, Näther C, Bensch W (2004) Eur J Inorg Chem (12):2553
Stephan H, Kanatzidis MG (1997) Inorg Chem 36:6050
Vaqueiro P, Darlow DP, Powell AV, Chippindale AM (2004) Solid State Ionics 172:601
Stähler R, Bensch W (2001) J Chem Soc Dalton Trans 2518
Stähler R, Mosel BD, Eckert H, Bensch W (2002) Angew Chem Int Ed 41:4487
Vaqueiro P, Chippindale AM, Powell AV (2004) Inorg Chem 43:7963
Bensch W, Näther C, Schur M (1997) Chem Commun 1773
Almsick T, Sheldrick WS (2006) Z Anorg Allg Chem 632:1413
Jia DX, Zhang Y, Zhao QX, Deng J (2006) Inorg Chem 45:9812
Fu ML, Guo GC, Cai LZ, Zhang ZJ, Huang JS (2005) Inorg Chem 44:184
Jia DX, Zhao QX, Dai J, Zhang Y, Zhu QY (2006) Z Anorg Allg Chem 632:349
Kromm A, Sheldrick WS (2008) Z Anorg Allg Chem 634:121
Kromm A, Sheldrick WS (2008) Z Anorg Allg Chem 634:225
Jia DX, Zhu QY, Dai J, Lu W, Guo WJ (2005) Inorg Chem 44:819
Jia DX, Zhao QX, Zhang Y, Dai J, Zou JL (2005) Inorg Chem 44:8861
Jia DX, Deng J, Zhao QX, Zhang Y (2007) J Mol Struct 833:114
Sheldrick GM (1997) SHELXS-97, program for structure solution. Universität of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, program for structure refinement. Universität of Göttingen, Germany
Neculai AM, Neculai D, Roesky HW, Magull J (2004) Polyhedron 23:183
Muller-Buschbaum K, Quitmann CC (2006) Inorg Chem 45:2678
Schaefer M, Engelke L, Bensch W (2003) Z Anorg Allg Chem 629:1912
Schur M, Bensch W (2000) Acta Cryst C56:1107
Schur M, Rijnberk H, Näther C, Bensch W (1998) Polyhedron 17:101
Cotton FA, Wilkinson G (1980) Advanced inorganic chemistry, 4th edn. Wiley, New York, p 1011
Acknowledgements
This work was supported by the National Natural Science Foundation of P. R. China (No. 20771077), and the Key Laboratory of Organic Synthesis of Jiangsu Province, Suzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Jj., Zhao, J., **, Qy. et al. Solvothermal Syntheses and Crystal Structures of Rare Earth Thioantimonates [Y(en)4]SbS4·0.5en and [Tm(en)4]SbS4·0.5en. J Chem Crystallogr 40, 975–980 (2010). https://doi.org/10.1007/s10870-010-9774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9774-y