Abstract
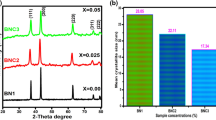
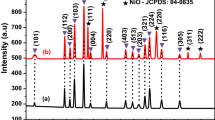
In this research paper, pristine nickel oxide (NiO) and Mn-doped nickel oxide nanoparticles (NPs) were manufactured through the chemical precipitation method. The X-ray diffraction (XRD), Fourier transform infrared (FT-IR), UV–Visible diffuse reflectance spectroscopy, scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDX), transmission electron microscopy/selected area electron diffraction (TEM/SAED), vibrating sample magnetometer, and cyclic voltammetry were used to examine the pristine and Mn-doped NiO NPs. The XRD results confirmed that all the samples exhibit face-centered cubic structures with decreasing crystallite sizes. SEM and TEM studies show NPs have spherical morphology. The deviation in-between the high energy bandgap and optical absorption has been investigated in pristine and Mn-doped samples. The presence of a peak in the FT-IR spectra at 446 cm−1 confirmed the creation of the NiO phase. Hysteresis measurements show the exchange of weak ferromagnetic to superparamagnetism in the samples due to incorporation of Mn ions. It has been confirmed that the highest capacitance was measured at 555 F/g with a 10 mV/s scan rate for pristine NiO samples. In this research, the pristine NiO and Mn-doped NiO NPs can be used in data storage and supercapacitor applications.









Similar content being viewed by others
Data availability
All the data are generated or analyzed during this study are included in this published article.
References
G. Wang, L. Zhang, J. Zhang, A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41(2), 797–828 (2012)
N.A. Mala, M.A. Dar, S. Sivakumar, S. Husain, K.M. Batoo, Enhanced electrochemical properties of zinc and manganese co-doped NiO nanostructures for its high-performance supercapacitor applications. Inorg. Chem. Commun. 142, 109661 (2022)
K. Sathishkumar, N. Shanmugam, N. Kannadasan, S. Cholan, G. Viruthagiri, Influence of Zn2+ ions incorporation on the magnetic and pseudo capacitance behaviors of NiO nanoparticles. Mater. Sci. Semicond. Process. 27, 846–853 (2014)
S. Sivakumar, N.A. Mala, K.M. Batoo, E.H. Raslan, Efficient, highly stable Zn2+ doped NiO nanoparticles with enhanced magnetic and supercapacitor applications. Mater. Technol. 37(10), 1375–1387 (2022)
S. Thota, J.H. Shim, M.S. Seehra, Size-dependent shifts of the Néel temperature and optical band-gap in NiO nanoparticles. J. Appl. Phys. 114(21), 214307 (2013)
Z. Li, L. Wei, Y. Liu, Y. Su, X. Dong, Y. Zhang, Facile synthesis of single-crystalline mesoporous NiO nanosheets as high-performance anode materials for Li-ion batteries. J. Mater. Sci.: Mater. Electron. 28(18), 13853–13860 (2017)
F.I. Dar, K.R. Moonoosawmy, M. Es-Souni, Morphology and property control of NiO nanostructures for supercapacitor applications. Nanoscale Res. Lett. 8(1), 1–7 (2013)
I. Hotovy, J. Huran, L. Spiess, S. Hascik, V. Rehacek, Preparation of nickel oxide thin films for gas sensors applications. Sens. Actuators B Chem. 57(1–3), 147–152 (1999)
C. Nie, W. Zeng, Y. Li, The 3D crystal morphologies of NiO gas sensor and constantly improved sensing properties to ethanol. J. Mater. Sci.: Mater. Electron. 30(2), 1794–1802 (2019)
M. Martini, G.E.D.S. Brito, M.C.D.A. Fantini, A.F. Craievich, A. Gorenstein, Electrochromic properties of NiO-based thin films prepared by sol–gel and dip coating. Electrochim. Acta 46(13–14), 2275–2279 (2001)
X.Y. Liu, Y.Q. Zhang, X.H. **a, S.J. Shi, Y. Lu, X.L. Wang et al., Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sources 239, 157–163 (2013)
H. Zhu, D.C. Rosenfeld, M. Harb, D.H. Anjum, M.N. Hedhili, S. Ould-Chikh, J.M. Basset, Ni–M–O (M= Sn, Ti, W) catalysts prepared by a dry mixing method for oxidative dehydrogenation of ethane. ACS Catal. 6(5), 2852–2866 (2016)
S. Sankar, S.K. Sharma, N. An, H. Lee, D.Y. Kim, Y.B. Im et al., Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik 127(22), 10727–10734 (2016)
B. Ebin, Simple preparation of Ni and NiO nanoparticles using raffinate solution originated from spent NiMH battery recycling. J. Inorg. Organomet. Polym Mater. 28(6), 2554–2563 (2018)
D. Hong, W. Yan, Q. Liu, T. Yao, Z. Sun, S. Wei, Structures and magnetic properties of Mn-doped NiO thin films. J. Phys. D Appl. Phys. 47(29), 295001 (2014)
M.J. Kartha, B.A. Reshi, P.S. Walke, D. Dastan, Morphological study of thin films: simulation and experimental insights using horizontal visibility graph. Ceram. Int. 48(4), 5066–5074 (2022)
Z.Y. Wang, R.H. Fan, Q.Q. Li, L. Qian, Preparation of NiO nanosheets by hydrothermal method and its electrochemical capacitive properties. Mater. Sci. Forum 848, 396–400 (2016)
B.A. Reshi, S. Kumar, A. Misra, R. Varma, Multivariable study on growth of diamond on diamond substrates by microwave plasma chemical vapour deposition. Mater. Res. Express 6(4), 046407 (2019)
K. Anandan, V. Rajendran, Effects of Mn on the magnetic and optical properties and photocatalytic activities of NiO nanoparticles synthesized via the simple precipitation process. Mater. Sci. Eng. B 199, 48–56 (2015)
C. Thangamani, P. Vijaya Kumar, K. Gurushankar, K. Pushpanathan, Structural and size dependence magnetic properties of Mn-doped NiO nanoparticles prepared by wet chemical method. J. Mater. Sci.: Mater. Electron. 31(14), 11101–11112 (2020)
N.A. Mala, M.A. Dar, S. Sivakumar, K.S. Bhat, G.N. Sinha, K.M. Batoo, Electrochemical supremacy of cobalt-doped nickel oxide and its supercapacitor applications with its mesoporous morphology. J. Mater. Sci.: Mater. Electron. 33(14), 11582–11590 (2022)
S. Sivakumar, N.A. Mala, Synthesis and characterization of manganese do** on NiO nanoparticles and its supercapacitor applications. Mater. Today: Proc. 49, 1469–1474 (2022)
K. Gopinadhan, S.C. Kashyap, D.K. Pandya, S. Chaudhary, High temperature ferromagnetism in Mn-doped SnO2 nanocrystalline thin films. J. Appl. Phys. 102(11), 113513 (2007)
M.A. Dar, D. Govindarajan, K.M. Batoo, M. Hadi, G.N. Dar, Photovoltaic and Supercapacitor performance of SnSe nanoparticles prepared through co-precipitation method. Mater. Technol. 37(10), 1396–1409 (2022)
R. Sathish Kumar, S. Johnson Jeyakumar, M. Jothibas, I. Kartharinal Punithavathy, J. Prince Richard, Influence of molar concentration on structural, optical and magnetic properties of NiO nanoparticles. J. Mater. Sci.: Mater. Electron. 28(20), 15668–15675 (2017)
M.A. Dar, D. Govindarajan, G.N. Dar, Comparing the electrochemical performance of bare SnS and Cr-doped sns nanoparticles synthesized through solvothermal method. Phys. Solid State 63(9), 1343–1350 (2021)
M.A. Dar, N.A. Mala, G.N. Dar, S.S. Kumar, D. Govindarajan, Structural, optical, antibacterial analysis of Se NPs synthesized by precipitation method. Adv. Nat. Sci.: Nanosci. Nanotechnol. 11(4), 045001 (2020)
M.A. Dar, D. Govindarajan, G.N. Dar, Facile synthesis of SnS nanostructures with different morphologies for supercapacitor and dye-sensitized solar cell applications. J. Mater. Sci.: Mater. Electron. 32(15), 20394–20409 (2021)
M.A. Dar, M.Y. Bhat, N.A. Mala, H.A. Rather, S. Venkatachalam, N. Srinivasan, Structural, morphological and supercapacitor applications of SnS nanomaterials prepared in three different types of solvents. Mater. Today: Proc. 66, 1689–1698 (2022)
M.A. Dar, D. Govindarajan, K.M. Batoo, C. Siva, Supercapacitor and magnetic properties of Fe doped SnS nanoparticles synthesized through solvothermal method. J. Energy Storage 52, 105034 (2022)
B.J. Rani, G. Ravi, R. Yuvakkumar, S. Ravichandran, F. Ameen, A. Al-Sabri, Efficient, highly stable Zn-doped NiO nanocluster electrocatalysts for electrochemical water splitting applications. J. Sol-Gel. Sci. Technol. 89(2), 500–510 (2019)
N.A. Mala, S. Sivakumar, K.M. Batoo, M. Hadi, Design and fabrication of iron-doped nickel oxide-based flexible electrode for high-performance energy storage applications. Inorg. Chem. Commun. 131, 108797 (2021)
Z. Ahmad, S. Kumar, C.K. Trinh, J.J. Shim, J.S. Lee, Decoupling electrochemical parameters of molecular-level-controlled polypyrrole and graphene oxide nanocomposite. Appl. Surf. Sci. 610, 155464 (2023)
Z. Ahmad, W.B. Kim, S. Kumar, T.H. Yoon, J.J. Shim, J.S. Lee, Redox-active supercapacitor electrode from two-monomer-connected precursor (Pyrrole: Anthraquinonedisulfonic acid: Pyrrole) and sulfonated multi-walled carbon nanotube. Electrochim. Acta 415, 140243 (2022)
B. Govindarajan, R. Palanimuthu, K.M. Manikandan, Influence of Mg do** in magnetic properties of NiO nanoparticles and its electrical applications. J. Mater. Sci.: Mater. Electron. 30(7), 6519–6527 (2019)
S. Sivakumar, N.A. Mala, K.M. Batoo, M.F. Ijaz, Conserved crystal phase and morphology: electrochemical supremacy of copper (Cu) and iron (Fe) dual-doped nickel oxide and its supercapacitor applications. Inorg. Chem. Commun. 134, 108959 (2021)
A. Jafari, S.P. Jahromi, K. Boustani, B.T. Goh, N.M. Huang, Evolution of structural and magnetic properties of nickel oxide nanoparticles: influence of annealing ambient and temperature. J. Magn. Magn. Mater. 469, 383–390 (2019)
G. Bharathy, P. Raji, Room temperature ferromagnetic behavior of Mn doped NiO nanoparticles: a suitable electrode material for supercapacitors. J. Mater. Sci.: Mater. Electron. 28(23), 17889–17895 (2017)
S. Farhadi, Z. Roostaei-Zaniyani, Simple and low-temperature synthesis of NiO nanoparticles through solid-state thermal decomposition of the hexa (ammine) Ni (II) nitrate,[Ni(NH3)6](NO3)2, complex. Polyhedron 30(7), 1244–1249 (2011)
S.J. Uke, S.P. Mardikar, D.R. Bambole, Y. Kumar, G.N. Chaudhari, Sol-gel citrate synthesized Zn doped MgFe2O4 nanocrystals: a promising supercapacitor electrode material. Mater. Sci. Energy Technol. 3, 446–455 (2020)
S. Kumar, I.A. Mir, Z. Ahmad, K. San Hui, D.A. Dinh, L. Zhu et al., Microflowers of Sn-Co-S derived from ultra-thin nanosheets for supercapacitor applications. J. Energy Storage 49, 104084 (2022)
G. Srikesh, A.S. Nesaraj, Facile preparation and characterization of novel manganese doped nickel oxide based nanostructured electrode materials for application in electrochemical supercapacitors. J. Asian Ceram. Soc. 8(3), 835–847 (2020)
Acknowledgments
NAM is grateful to the Head of the Department of Physics at Annamalai University for providing the required resources to complete this work.
Author information
Authors and Affiliations
Contributions
SS contributed to supervision, and visualization; NAM contributed to methodology, validation, conceptualization, formal analysis, visualization, writing of the original draft, and writing, reviewing, & editing of the manuscript; MAD, MDR, KMB, BAR, and ZA contributed to reviewing, & editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mala, N.A., Dar, M.A., Rather, M.u.D. et al. Supercapacitor and magnetic properties of NiO and manganese-doped NiO nanoparticles synthesized by chemical precipitation method. J Mater Sci: Mater Electron 34, 505 (2023). https://doi.org/10.1007/s10854-023-09907-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-09907-5