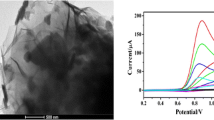
Abstract
Electrochemical sensing is one of the most powerful tools used for the detection of toxic reagents or for examining health issues. In the present work, we have studied the effect of reduced graphene oxide (RGO) in MoS2-RGO nanocomposite for sensing of ascorbic acid. For synthesizing MoS2-RGO nanocomposite, an in-situ microwave-assisted technique has been used and further confirmation of the formation of nanocomposites is done by using field emission scanning electron microscopy, X-ray diffraction, and Raman Spectroscopy. Electrochemical measurements are taken by cyclic voltammetry, differential pulse voltammetry (DPV), and impedance spectroscopy (EIS). Designed sensing material has displayed a noticeable linear increase of current with the concentration of the analyte. It has shown a very precise limit of detection of ~ 0.43 µM/0.16 μM and a remarkable sensitivity of ~ 2.9 µAµM−1 cm−2/1508 Ω μM−1 cm−2 with DPV/EIS. An inductive behavior observed in the Nyquist plots, due to the intercalation process on the surface of the electrode and dissociation of analyte in the buffer solution, was found responsible for the remarkable sensing demeanor of MoS2-RGO@GCE towards ascorbic-acid.









Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and the datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
S. Bilal, A. Akbar, A.-U.-H.A. Shah, Highly selective and reproducible electrochemical sensing of ascorbic acid through a conductive polymer coated electrode. Polymers 11, 1346 (2019)
S. Li, Y. Ma, Y. Liu, G. **n, M. Wang, Z. Zhang, Z. Liu, Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid. RSC Adv. 9(6), 2997–3003 (2019)
A.K. Barui, R. Sharma, Y.S. Rajput, S. Singh, A rapid paper chromatographic method for detection of anionic detergent in milk. J. Food Sci. Technol. 50, 826–829 (2013)
J. Dostálek, J. Přibyl, J. Homola, P. Skládal, Multichannel SPR biosensor for detection of endocrine-disrupting compounds. Anal. Bioanal. Chem. 389, 1841–1847 (2007)
G.A. Ibañez, G.M. Escandar, Luminescence sensors applied to water analysis of organic pollutants—an update. Sensors 11, 11081–11102 (2011)
A. Mishra, R. Dheepika, P.A. Parvathy, P.M. Imran, N.S.P. Bhuvanesh, S. Nagarajan, Fluorescence quenching based detection of nitroaromatics using luminescent triphenylamine carboxylic acids. Sci. Rep. 11, 1–10 (2021)
X. Zhang, Y. Cao, S. Yu, F. Yang, P. **, An electrochemical biosensor for ascorbic acid based on carbon-supported PdNi nanoparticles. Biosens. Bioelectron. 44, 183–190 (2013)
J. Yan, S. Liu, Z. Zhang, G. He, P. Zhou, H. Liang, L. Tian, X. Zhou, H. Jiang, Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on graphene anchored with Pd–Pt nanoparticles. Colloids Surf. B 111, 392–397 (2013)
C. Zhou, S. Li, W. Zhu, H. Pang, H. Ma, A sensor of a polyoxometalate and Au–Pd alloy for simultaneously detection of dopamine and ascorbic acid. Electrochim. Acta 113, 454–463 (2013)
D. Li, X. Liu, R. Yi, J. Zhang, Z. Su, G. Wei, Electrochemical sensor based on novel two-dimensional nanohybrids: MoS2 nanosheets conjugated with organic copper nanowires for simultaneous detection of hydrogen peroxide and ascorbic acid. Inorg. Chem. Front. 5, 112–119 (2018)
A. Sinha, Dhanjai, B. Tan, Y. Huang, H. Zhao, X. Dang, J. Chen, R. Jain, MoS2 nanostructures for electrochemical sensing of multidisciplinary targets: a review. TrAC Trends Anal. Chem. 102, 75–90 (2018)
E. Akbari, K. Jahanbin, A. Afroozeh, P. Yupapin, Z. Buntat, Brief review of monolayer molybdenum disulfide application in gas sensor. Physica B 545, 510–518 (2018)
J.-W. Jiang, Graphene versus MoS2: a short review. Front. Phys. 10, 287–302 (2015)
N.A. Kumar, M.A. Dar, R. Gul, J.-B. Baek, Graphene and molybdenum disulfide hybrids: synthesis and applications. Mater. Today 18, 286–298 (2015)
F. Wypych, T. Weber, R. Prins, Scanning tunneling microscopic investigation of Kx(H2O)yMoS2. Surf. Sci. 380, L474–L478 (1997)
X. Gan, L.Y.S. Lee, K.-Y. Wong, T.W. Lo, K.H. Ho, D.Y. Lei, H. Zhao, 2H/1T phase transition of multilayer MoS2 by electrochemical incorporation of S vacancies. ACS Appl. Energy Mater. 1, 4754–4765 (2018)
S.H. El-Mahalawy, B.L. Evans, The thermal expansion of 2H-MoS2, 2H-MoSe2 and 2H-WSe2 between 20 and 800°C. J. Appl. Crystallogr. 9, 403–406 (1976)
R.M.A. Khalil, F. Hussain, A.M. Rana, M. Imran, G. Murtaza, Comparative study of polytype 2H-MoS2 and 3R-MoS2 systems by employing DFT. Physica E: Low-dimens. Syst. Nanostruct. 106, 338–345 (2019)
Y. Tokura, N. Nagaosa, Orbital physics in transition-metal oxides. Science 288, 462–468 (2000)
F. Chen, W. Su, S. Zhao, Y. Lv, S. Ding, L. Fu, Morphological evolution of atomically thin MoS2 flakes synthesized by a chemical vapor deposition strategy. CrystEngComm 22, 4174–4179 (2020)
H. Gómez-Pozos, J. González-Vidal, G. Torres, M. Olvera, L. Castañeda, Physical characterization and effect of effective surface area on the sensing properties of tin dioxide thin solid films in a propane atmosphere. Sensors 14, 403–415 (2013)
A.K. Gautam, M. Faraz, N. Khare, Enhanced thermoelectric properties of MoS2 with the incorporation of reduced graphene oxide (RGO). J. Alloys Compd. 838, 155673 (2020)
D. Gupta, V. Chauhan, R. Kumar, A comprehensive review on synthesis and applications of molybdenum disulfide (MoS2) material: past and recent developments. Inorg. Chem. Commun. 121, 108200 (2020)
G. Solomon, R. Mazzaro, V. Morandi, I. Concina, A. Vomiero, Microwave-assisted vs. conventional hydrothermal synthesis of MoS2 nanosheets: application towards hydrogen evolution reaction. Curr. Comput.-Aided Drug Des. 10, 1040 (2020)
J. Kudr, V. Adam, O. Zitka, Fabrication of graphene/molybdenum disulfide composites and their usage as actuators for electrochemical sensors and biosensors. Molecules 24, 3374 (2019)
B. Rawat, K.K. Mishra, U. Barman, L. Arora, D. Pal, R.P. Paily, Two-dimensional MoS2-based electrochemical biosensor for highly selective detection of glutathione. IEEE Sens. J. 20, 6937–6944 (2020)
X. Qiao, K. Li, J. Xu, N. Cheng, Q. Sheng, W. Cao, T. Yue, J. Zheng, Novel electrochemical sensing platform for ultrasensitive detection of cardiac troponin I based on aptamer-MoS2 nanoconjugates. Biosens. Bioelectron. 113, 142–147 (2018)
R. Aswathi, K.Y. Sandhya, Ultrasensitive and selective electrochemical sensing of Hg(ii) ions in normal and sea water using solvent exfoliated MoS2: affinity matters. J. Mater. Chem. A 6, 14602–14613 (2018)
M.D. Petit-Domínguez, C. Quintana, L. Vázquez, M. del Pozo, I. Cuadrado, A.M. Parra-Alfambra, E. Casero, Synergistic effect of MoS2 and diamond nanoparticles in electrochemical sensors: determination of the anticonvulsant drug valproic acid. Microchim. Acta 185, 1–10 (2018)
S.Y. Park, J.E. Lee, Y.H. Kim, J.J. Kim, Y.-S. Shim, S.Y. Kim, M.H. Lee, H.W. Jang, Room temperature humidity sensors based on rGO/MoS2 hybrid composites synthesized by hydrothermal method. Sens. Actuators B Chem. 258, 775–782 (2018)
H.-Y. Chen, J. Wang, L. Meng, T. Yang, K. Jiao, Thin-layered MoS2/polyaniline nanocomposite for highly sensitive electrochemical detection of chloramphenicol. Chin. Chem. Lett. 27, 231–234 (2016)
Y. Yang, Q. Lei, J. Li, C. Hong, Z. Zhao, H. Xu, J. Hu, Synthesis and enhanced electrochemical properties of AuNPs@MoS2/rGO hybrid structures for highly sensitive nitrite detection. Microchem. J. 172, 106904 (2022)
R. Sha, N. Vishnu, S. Badhulika, MoS2 based ultra-low-cost, flexible, non-enzymatic and non-invasive electrochemical sensor for highly selective detection of Uric acid in human urine samples. Sens. Actuators B Chem. 279, 53–60 (2019)
L. **ng, Z. Ma, A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim. Acta 183, 257–263 (2015)
N.I. Zaaba, K.L. Foo, U. Hashim, S.J. Tan, W.-W. Liu, C.H. Voon, Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng. 184, 469–477 (2017)
C. Lee, H. Yan, L.E. Brus, T.F. Heinz, J. Hone, S. Ryu, Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 4, 2695–2700 (2010)
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prud’homme, I.A. Aksay, R. Car, Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8, 36–41 (2007)
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, R.S. Ruoff, Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007)
H.-Y. He, One-step assembly of 2H–1T MoS2:Cu/reduced graphene oxide nanosheets for highly efficient hydrogen evolution. Sci. Rep. 7, 1–7 (2017)
R. Wang, S. Gao, K. Wang, M. Zhou, S. Cheng, K. Jiang, MoS2@rGO nanoflakes as high performance anode materials in sodium ion batteries. Sci. Rep. 7, 1–9 (2017)
N. Karikalan, R. Karthik, S.-M. Chen, H.-A. Chen, A voltammetric determination of caffeic acid in red wines based on the nitrogen doped carbon modified glassy carbon electrode. Sci. Rep. 7, 1–10 (2017)
A.A. Odeh, Y. Al-Douri, C.H. Voon, R.M. Ayub, S.C.B. Gopinath, R.A. Odeh, M. Ameri, A. Bouhemadou, A needle-like Cu2CdSnS4 alloy nanostructure-based integrated electrochemical biosensor for detecting the DNA of Dengue serotype 2. Microchim. Acta 184, 2211–2218 (2017)
Y. Wei, Y. Liu, Z. Xu, S. Wang, B. Chen, D. Zhang, Y. Fang, Simultaneous detection of ascorbic acid, dopamine, and uric acid using a novel electrochemical sensor based on palladium nanoparticles/reduced graphene oxide nanocomposite. Int. J. Anal. Chem. 2020, 1–13 (2020)
J. Bisquert, H. Randriamahazaka, G. Garcia-Belmonte, Inductive behaviour by charge-transfer and relaxation in solid-state electrochemistry. Electrochim. Acta 51, 627–640 (2005)
M. Saraf, K. Natarajan, S.M. Mobin, Emerging robust heterostructure of MoS2–rGO for high-performance supercapacitors. ACS Appl. Mater. Interfaces 10, 16588–16595 (2018)
X. Fan, Z. Li, S. Wang, L. Liu, P. Liu, F. Chen, X. Zheng, Electrochemical impedance biosensor for the determination of lipopolysaccharide using peptide as the recognition molecule. J Braz Chem Soc 30, 1762–1768 (2019)
H. Sun, J. Chao, X. Zuo, S. Su, X. Liu, L. Yuwen, C. Fan, L. Wang, Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 4, 27625 (2014)
M. Huang, Electrochemical sensor for detection of ascorbic acid based on MoS2-AuNPs modified glassy carbon electrode. Int. J. Electrochem. Sci. 16, 151014 (2021)
A. Gülden, H. Çelikkan, ASKORBİK ASİTİN MoS2 ESASLI ELEKTROTLA ELEKTROKİMYASAL TAYİNİ. Gazi Üniversitesi Mühendislik-Mimarlık Fakültesi Dergisi (2017). https://doi.org/10.17341/gazimmfd.337608
M. Saraf, K. Natarajan, A.K. Saini, S.M. Mobin, Small biomolecule sensors based on an innovative MoS2–rGO heterostructure modified electrode platform: a binder-free approach. Dalton Trans. 46, 15848–15858 (2017)
Acknowledgements
The authors extend their gratitude to Central Instrumentation Facility, Division of Research and Development, Lovely Professional University for providing research facilities. The funds provided by Lovely Professional University, under the scheme LPU/DRDSEED/SAC/65 are also acknowledged.
Author information
Authors and Affiliations
Contributions
SS synthesized the material and done its optimization. PK made substantial contributions towards conceptualization and designing of the problem. SJ is responsible for the acquisition, analysis, and interpretation of data. KSS articulated and supervised the problem.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Kumar, P., Jabeen, S. et al. Hierarchical granular morphology of MoS2-RGO nanocomposite for electrochemical sensing of ascorbic-acid. J Mater Sci: Mater Electron 33, 21048–21059 (2022). https://doi.org/10.1007/s10854-022-08909-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08909-z