Abstract
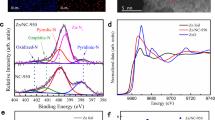
The development of economic catalysts for aerobic oxidation procedure has attracted extensive attention. In this work, a novel Ni/Zn supported defective carbon with multi-functional catalytic sites was fabricated via a two-step pyrolysis-H2O2 treatment. The catalyst was applied to the Baeyer–Villiger (B–V) oxidation using ambient air as a green and safe oxidant. The catalyst with optimal Ni/Zn ratio of (2:1) delivers a high catalytic activity (> 92%) and perfect selectivity (> 99%) for the conversion of a wide range of substituted cyclic-ketones to the corresponding lactones. The characterization results have clarified that the H2O2 treatment leads to the formation of active N/O-group on the catalyst surface, which facilitates the adsorption of substrate/intermediate molecules and benefits the reaction. Moreover, the synergistic effect between multi-functional sites results in the buffering/stabilizing of free radicals, enhanced efficiency of oxygen insertion to form lactone. The design principle in this work is believed to shed light on the exploration of all-in-one solid catalyst for diverse oxidative reaction.
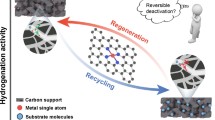
Graphical Abstract










Similar content being viewed by others
References
Chamas A, Moon H, Zheng J et al (2020) ACS Sus Chem Engin 8:3494. https://doi.org/10.1021/acssuschemeng.9b06635
Wei R, Tiso T, Bertling J, O’Connor K, Blank LM, Bornscheuer UT (2020) Nat Catal 3:867. https://doi.org/10.1038/s41929-020-00521-w
Meereboer KW, Misra M, Mohanty AK (2020) Green Chem 22:5519. https://doi.org/10.1039/d0gc01647k
Filiciotto L, Rothenberg G (2021) Chemsuschem 14:56. https://doi.org/10.1002/cssc.202002044
Uyanik M, Ishihara K (2013) ACS Catal 3:513. https://doi.org/10.1021/cs300821u
Baeyer A, Villiger V (1899) Ber Dtsch Chem Ges 32:3625. https://doi.org/10.1002/cber.189903203151
Renz M, Meunier B (1999) Eur J Organ Chem 1999:737. https://doi.org/10.1002/(SICI)1099-0690(199904)1999
Brink GJ, Arends I, Sheldon RA (2004) Chem Rev 104:4105. https://doi.org/10.1021/cr030011l
Liu C, Wen KG, Zeng XP, Peng YY (2020) Adv Syn Catal 362:1015. https://doi.org/10.1002/adsc.201901178
Fürst MJLJ, Gran-Scheuch A, Aalbers FS, Fraaije MW (2019) ACS Catal 2019:11207. https://doi.org/10.1021/acscatal.9b03396
Markiton M, Boncel S, Janas D, Chrobok A (2016) ACS Sus Chem Engin 5:1685. https://doi.org/10.1021/acssuschemeng.6b02433
Leisch H, Morley K, Lau PC (2011) Chem Rev 111:4165. https://doi.org/10.1021/cr1003437
Jeong E-Y, Ansari MB, Park S-E (2011) ACS Catal 1:855. https://doi.org/10.1021/cs200163r
Chen S, Zhou X, Li Y, Luo R, Ji H (2014) Chem Engin J 241:138. https://doi.org/10.1016/j.cej.2013.12.027
Rahman S, Enjamuri N, Gomes R et al (2015) Appl Catal A: Gen 505:515. https://doi.org/10.1016/j.apcata.2015.03.014
Tang Z, **ao J, Li F et al (2020) ACS Omega 5:10451. https://doi.org/10.1021/acsomega.0c00443
**ao H, Shin H, Goddard WA 3rd (2018) Proc Natl Acad Sci USA 115:5872. https://doi.org/10.1073/pnas.1722034115
** Y, Huang S, Yue X, Du H, Shen PK (2018) ACS Catal 8:2359. https://doi.org/10.1021/acscatal.7b04226
Zheng C, Chang S, Yang C et al (2018) Tetrahedron 74:2608. https://doi.org/10.1016/j.tet.2018.04.009
Huo H, Wu L, Ma J et al (2016) ChemCatChem 8:779. https://doi.org/10.1002/cctc.201501107
Liu Z, Zhou Z, Qin J, Liu G, Huang H, Wu W (2018) ChemistrySelect 3:6434. https://doi.org/10.1002/slct.201801247
Zhou Z, Wang J, Qin J, Yu Y, Wu W (2017) J Porous Mater 25:835. https://doi.org/10.1007/s10934-017-0496-9
Li Y-F, Guo M-Q, Yin S-F et al (2013) Carbon 55:269. https://doi.org/10.1016/j.carbon.2012.12.036
Nabae Y, Rokubuichi H, Mikuni M, Kuang Y, Hayakawa T, Kakimoto M-a (2013) Catalysis by carbon materials for the aerobic baeyer-villiger oxidation in the presence of aldehydes. ACS Catal 3(2):230–236
Chen S-Y, Zhou X-T, Wang J-X et al (2017) Mol Catal 438:152. https://doi.org/10.1016/j.mcat.2017.06.001
Wei Z, Wang J, Mao S et al (2015) ACS Catal 5:4783. https://doi.org/10.1021/acscatal.5b00737
Hu M, Reboul J, Furukawa S et al (2012) J Am Chem Soc 134:2864. https://doi.org/10.1021/ja208940u
Zhong G, Li S, Xu S, Liao W, Fu X, Peng F (2018) ACS Sus Chem Engin 6:15108. https://doi.org/10.1021/acssuschemeng.8b03582
Wang Y, Liu Y, Liu W, Chen H, Zhang G, Wang J (2015) Mater Lett 154:64. https://doi.org/10.1016/j.matlet.2015.04.073
Wang X, Blechert S, Antonietti M (2012) ACS Catal 2:1596. https://doi.org/10.1021/cs300240x
Zhang M, Gao B (2013) Chem Engin J 226:286. https://doi.org/10.1016/j.cej.2013.04.077
Peng Y, Liu HW (2006) Ind Engin Chem Res 45:6483. https://doi.org/10.1021/ie0604627
Donoeva B, Masoud N, de Jongh PE (2017) ACS Catal 7:4581. https://doi.org/10.1021/acscatal.7b00829
Yang X, Wan Y, Zheng Y et al (2019) Chem Engin J 366:608. https://doi.org/10.1016/j.cej.2019.02.119
**a J, He G, Zhang L, Sun X, Wang X (2016) Appl Catal B: Environ 180:408. https://doi.org/10.1016/j.apcatb.2015.06.043
Wang Y, Ren N, ** J et al (2021) ACS ES&T Engin 1:32. https://doi.org/10.1021/acsestengg.0c00004
Zhang X, Yang H, Yang G, Li S, Wang X, Ma J (2018) ACS Sus Chem & Engin 6:5868. https://doi.org/10.1021/acssuschemeng.7b04167
Sun M, Liu H-H, Tao X-F, Zhai L-F, Wang S (2021) ACS ES&T Engin 1:173. https://doi.org/10.1021/acsestengg.0c00036
Li J, Shen B, Hong Z, Lin B, Gao B, Chen Y (2012) Chem Comm 48:12017. https://doi.org/10.1039/C2CC35862J
Wan X, Zhou C, Chen J et al (2014) ACS Catal 4:2175. https://doi.org/10.1021/cs5003096
He L, Weniger F, Neumann H, Beller M (2016) Angew Chem Int Ed 55:12582. https://doi.org/10.1002/anie.201603198
Cao Y, Mao S, Li M, Chen Y, Wang Y (2017) ACS Catal 7:8090. https://doi.org/10.1021/acscatal.7b02335
Deng H, Li Q, Liu J, Wang F (2017) Carbon 112:219. https://doi.org/10.1016/j.carbon.2016.11.014
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21773195). Y.S. Sun also thanks the financial support from Guangdong Basic and Applied Basic Research Foundation (2020A1515110904), State Key Laboratory of Physical Chemistry of Solid Surfaces, **amen University and Nanqiang Young Top-notch Talent Fellowship from **amen University.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, X., Li, B., Shi, K. et al. Ni-Zn supported defective carbon with multi-functional catalytic sites for Baeyer–Villiger reaction using air as oxidant. J Mater Sci 56, 14684–14699 (2021). https://doi.org/10.1007/s10853-021-06197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06197-2