Abstract
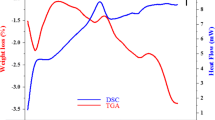
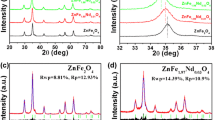
This paper firstly studied an obvious magnetic transformation from paramagnetism to superparamagnetism in hydrogenated ZnFe2O4 nanoparticles. The zinc ferrite nanoparticles were successfully prepared by a simple solid-state reaction followed by a ball milling method. Moderate thermal treatments under hydrogen atmosphere were carried out, and a phenomenon was observed that the magnetic behavior of zinc ferrite nanoparticles transformed from paramagnetism to superparamagnetism. Various characterizations were carried out to confirm the creation of the hydrogen do** induced defects under post-treatments, which may be crucial for the magnetic transformation. By studying the effect of the defects caused by hydrogenation on the magnetization, we found that the oxygen vacancies could alter the magnetic ordering of materials and therefore modulate the magnetic behaviors. In addition, the saturation magnetization decreased after reheating the hydrogenated Zn-ferrite samples in the air, which indicated that the oxygen vacancies and Fe2+ ions directly modulated the magnetization of the Zn-ferrite materials. Notably, the residual saturation magnetization was still higher than raw Zn-ferrite, indicating that the changes of cation distribution could also modulate the magnetization and certain changes were irreversible.







Similar content being viewed by others
References
Granone LI, Ulpe AC, Robben L, Klimke S, Jahns M, Renz F, Gesing TM, Bredow T, Dillert R, Bahnemann DW (2018) Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys Chem Chem Phys 20:28267–28278
Liu C, Peng T, Wang C, Lu Y, Yan H, Luo Y (2017) Three-dimensional ZnFe2O4@MnO2 hierarchical core/shell nanosheet arrays as high-performance battery-type electrode materials. J Alloys Compd 720:86–94
Amiri M, Salavati-Niasari M, Akbari A (2019) Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv Colloid Interface Sci 265:29–44
Bini M, Tondo C, Capsoni D, Mozzati MC, Albini B, Galinetto P (2018) Superparamagnetic ZnFe2O4 nanoparticles: the effect of Ca and Gd do**. Mater Chem Phys 204:72–82
Biswas S, Panja SS, Bose S (2018) Physical insight into the mechanism of electromagnetic shielding in polymer nanocomposites containing multiwalled carbon nanotubes and inverse-spinel ferrites. J Phys Chem C 122:19425–19437
Sharma R, Thakur P, Sharma P, Sharma V (2017) Ferrimagnetic Ni2+ doped Mg–Zn spinel ferrite nanoparticles for high density information storage. J Alloys Compd 704:7–17
El-Remaily MAEA, Ali A, Abu-Dief AM (2015) CuFe2O4 nanoparticles: an efficient heterogeneous magnetically separable catalyst for synthesis of some novel propynyl-1H-imidazoles derivatives. Tetrahedron 71:2579–2584
Abu-Dief AM, Nassar IF, Elsayed WH (2016) Magnetic NiFe2O4 nanoparticles: efficient, heterogeneous and reusable catalyst for synthesis of acetylferrocene chalcones and their anti-tumour activity. Appl Organomet Chem 30:917–923
Marzouk AA, Abu-Dief AM, Abdelhamid AA (2018) Hydrothermal preparation and characterization of ZnFe2O4 magnetic nanoparticles as an efficient heterogeneous catalyst for the synthesis of multi-substituted imidazoles and study of their anti-inflammatory activity. Appl Organomet Chem 32(1):3794
Melo Quintero JJ, Salcedo Rodriguez KL, Rodriguez Torres CE, Errico LA (2019) Ab initio study of the role of defects on the magnetic response and the structural, electronic and hyperfine properties of ZnFe2O4. J Alloys Compd 775:1117–1128
Mathew DS, Juang R-S (2007) An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem Eng J 129:51–65
Laokul P, Amornkitbamrung V, Seraphin S, Maensiri S (2011) Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr Appl Phys 11:101–108
Rodriguez Torres CE, Golmar F, Ziese M, Esquinazi P, Heluani SP (2011) Evidence of defect-induced ferromagnetism in ZnFe2O4 thin films. Phys Rev B 84(1–5):064404
Blanco-Gutierrez V, Urones-Garrote E, Torralvo-Fernandez MJ, Saez-Puche R (2010) ZnFe2O4 nanoparticles: different magnetic behavior when they are hosted in porous structures. Chem Mater 22:6130–6137
Deraz NM (2011) Fabrication, characterization and magnetic behaviour of alumina-doped zinc ferrite nano-particles. J Anal Appl Pyrolysis 91:8–54
Zhang Y, Chen Y, Kou Q, Wang Z, Han D, Sun Y, Yang J, Liu Y, Yang L (2018) Effects of Nd concentration on structural and magnetic properties of ZnFe2O4 nanoparticles. J Mater Sci Mater Electron 29:3665–3671
Pendyala SK, Thyagarajan K, Kumar AG, Obulapathi L (2018) Effect of Mg do** on physical properties of Zn ferrite nanoparticles. J Aust Ceram Soc 54:467–473
Heiba ZK, Mohamed MB, Wahba AM (2016) Effect of Mo substitution on structural and magnetic properties of Zinc ferrite nanoparticles. J Mol Struct 1108:347–351
Thankachan RM, Cyriac J, Raneesh B, Kalarikkal N, Sanyal D, Nambissan PMG (2015) Cr3+-substitution induced structural reconfigurations in the nanocrystalline spinel compound ZnFe2O4 as revealed from X-ray diffraction, positron annihilation and Mossbauer spectroscopic studies. RSC Adv 5:64966–64975
Nam PH, Phuc NX, Linh PH, Lu LT, Manh DH, Phong PT, Lee I-J (2018) Effect of zinc on structure, optical and magnetic properties and magnetic heating efficiency of Mn1−xZnxFe2O4 nanoparticles. Phys B 550:428–435
Phan TL, Tran N, Kim DH, Dang NT, Manh DH, Bach TN, Liu CL, Lee BW (2017) Magnetic and magnetocaloric properties of Zn1−xCoxFe2O4 nanoparticles. J Electron Mater 46:4214–4226
Mehran E, Shayesteh SF, Nasehnia F (2016) Investigation of structural and magnetic effects of cobalt do** in ZnFe2O4 nanoparticles. J Supercond Nov Magn 29:1241–1247
Rodriguez Torres CE, Pasquevich GA, Mendoza Zelis P, Golmar F, Heluani SP et al (2014) Oxygen-vacancy-induced local ferromagnetism as a driving mechanism in enhancing the magnetic response of ferrites. Phys Rev B 89:104411
Quintero JM, Rodriguez KLS, Pasquevich GA, Zelis PM, Stewart SJ, Rodriguez Torres CE, Errico LA (2016) Experimental and ab initio study of the hyperfine parameters of ZnFe2O4 with defects. Hyperfine Interact 237:63
Wang GM, Yang Y, Han DD, Li Y (2017) Oxygen defective metal oxides for energy conversion and storage. Nano Today 13:23–39
Zhu X, Guijarro N, Liu Y, Schouwink P, Wells RA, Le Formal F, Sun S, Gao C, Sivula K (2018) Spinel structural disorder influences solar–water-splitting performance of ZnFe2O4 nanorod photoanodes. Adv Mater 30:1801612
Babu KR, Singh R (2018) Effect of RF power on structural, magnetic, and optical properties of CoFe2O4 thin films. J Supercond Nov Magn 31:4029–4037
Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM (2016) Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: sonochemical synthesis, characterization DNA interaction, in vitro antimicrobial and anticancer activities. Bioorgan Chem 69:140–152
Abu-Dief AM, Mahmoud WS (2017) α-Bi2O3 nanorods: synthesis, characterization and UV-photocatalytic activity. Mater Res Express 4:35–39
Mahmoud WS, Abu-Dief AM (2018) Synthesis, characterization and photocatalysis enhancement of Eu2O3–ZnO mixed oxide nanoparticles. J Phys Chem Solids 116:375–385
Atif M, Hasanain SK, Nadeem M (2006) Magnetization of sol–gel prepared zinc ferrite nanoparticles: effects of inversion and particle size. Solid State Commun 138:416–421
Yang T, Xue J, Tan H, **e A, Li S, Yan W, Shen Y (2018) Highly ordered ZnO/ZnFe2O4 inverse opals with binder-free heterojunction interfaces for high-performance photoelectrochemical water splitting. J Mater Chem A 6:1210–1218
Sapna, Budhiraja N, Kumar V, Singh SK (2019) Shape-controlled synthesis of superparamagnetic ZnFe2O4 hierarchical structures and their comparative structural, optical and magnetic properties. Ceram Int 45:1067–1076
Wang ZW, Schiferl D, Zhao YS, O’Neill HSC (2003) High pressure Raman spectroscopy of spinel-type ferrite ZnFe2O4. J Phys Chem Solid 64:2517–2523
Fu Y, Wang X (2011) Magnetically separable ZnFe2O4-graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind Eng Chem Res 50:7210–7218
Li Y, Li Y, Xu X, Ding C, Chen N, Ding H, Lu A (2019) Structural disorder controlled oxygen vacancy and photocatalytic activity of spinel-type minerals: a case study of ZnFe2O4. Chem Geol 504:276–287
Yu K, Lei D, Feng Y, Yu H, Chang Y, Wang Y, Liu Y, Wang G-C, Lou L-L, Liu S, Zhou W (2018) The role of Bi-do** in promoting electron transfer and catalytic performance of Pt/3DOM-Ce1−xBiO2−δ. J Catal 365:292–302
Zhan S, Zhang H, Zhang Y, Shi Q, Li Y, Li X (2017) Efficient NH3–SCR removal of NOx with highly ordered mesoporous WO3(χ)–CeO2 at low temperatures. Appl Catal B Environ 203:199–209
Leconte N, Soriano D, Roche S, Ordejon P, Charlier J-C, Palacios JJ (2011) Magnetism-dependent transport phenomena in hydrogenated graphene: from spin-splitting to localization effects. ACS Nano 5:3987–3992
Lv H, Ma L, Zeng P, Ke D, Peng T (2010) Synthesis of floriated ZnFe2O4 with porous nanorod structures and its photocatalytic hydrogen production under visible light. J Mater Chem 20:3665–3672
Chakraborty M, Thangavel R, Biswas A, Udayabhanu G (2016) Facile synthesis, and the optical and electrical properties of nanocrystalline ZnFe2O4 thin films. CrystEngComm 18:3095–3103
Ayyappan S, Paneerselvam G, Antony MP, Philip J (2011) Structural stability of ZnFe2O4 nanoparticles under different annealing conditions. Mater Chem Phys 128:400–404
Hamdeh HH, Ho JC, Oliver SA, Willey RJ, Oliveri G, Busca G (1997) Magnetic properties of partially-inverted zinc ferrite aerogel powders. J Appl Phys 81:1851–1857
Abu-Dief AM, Abdelbaky MSM, Martínez-Blanco D, Amghouz Z, García-Granda S (2016) Effect of chromium substitution on the structural and magnetic properties of nanocrystalline zinc ferrite. Mater Chem Phys 174:164–171
Ibrahima EMM, Abdel-Rahman LH, Abudief AM, Elshafaie A, Hamdan SK, Ahmed AM (2018) Electric, thermoelectric and magnetic characterization of γ-Fe2O3 and Co3O4 nanoparticles synthesized by facile thermal decomposition of metal–Schiff base complexes. Mater Res Bull 99:103–108
Anumol CN, Chithra M, Shalini MG, Sahoo SC (2019) Effect of annealing on structural and magnetic properties of NiFe2O4/ZnFe2O4 nanocomposites. J Magn Magn Mater 469:81–88
Bullita S, Casu A, Casula MF, Concas G, Congiu F, Corrias A, Falqui A, Loche D, Marras C (2014) ZnFe2O4 nanoparticles dispersed in a highly porous silica aerogel matrix: a magnetic study. Phys Chem Chem Phys 16:4843–4852
Rodriguez KLS, Stewart SJ, Zelis PMM, Pasquevich GA, Torres CER (2018) Role of defects on the magnetic behaviour of the geometrically frustrated spinel ZnFe2O4. J Alloys Compd 752:289–295
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21777080); the Specialized Fund for the Fundamental Research Funds for the Central Universities (Grant No. FR-FTP-17-084A1) and fund of State Key Laboratory of New Ceramic and Fine Processing Tsinghua University (No. KF201815).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
In this paper, all the authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, Y., Shi, J., Tang, Y. et al. Investigation of significant magnetic transformation for hydrogenated ZnFe2O4 nanoparticles. J Mater Sci 55, 1464–1474 (2020). https://doi.org/10.1007/s10853-019-04053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04053-y