Abstract
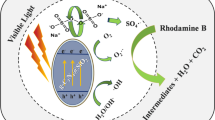
The catalytic activities of three topotactic phase strontium cobaltites (SCO, hexagonal, orthorhombic and tetragonal) for H2O2 decomposition were studied. All they present excellent catalytic activity with good stability at cycled test, and the mesoporous tetragonal SCO exhibits the best activity. Detailed kinetic research indicates the zero-order process of H2O2 decomposition. Increasing the temperature will accelerate the reactions greatly with the controlled temperature in the range of 20–45 °C. The calculated active energy (Ea) for H-SCO, O-SCO and T-SCO is 93.2, 85.1 and 87.5 (kJ/mol), respectively. The efficient catalytic degradation of Rhodamine B illustrates that SCO is a kind of promising catalysis for the advanced oxidation process.







Similar content being viewed by others
References
Tahini HA, Tan X, Schwingenschlögl U, Smith SC (2016) Formation and migration of oxygen vacancies in SrCoO3 and their effect on oxygen evolution reactions. ACS Catal 6:5565–5570
Hu S, Wang Y, Cazorla C, Seidel J (2017) Strain-enhanced oxygen dynamics and redox reversibility in topotactic SrCoO3−δ (0 < δ ≤ 0.5). Chem Mater 29:708–717
Jeen H, Choi WS, Freeland JW, Ohta H, Jung CU, Lee HN (2013) Topotactic phase transformation of the brownmillerite SrCoO2.5 to the perovskite SrCoO3−δ. Adv Mater 25:3651–3656
Petrie JR, Mitra C, Jeen H, Choi WS, Meyer TL, Reboredo FA, Freeland JW, Eres G, Lee HN (2016) Strain control of oxygen vacancies in epitaxial strontium cobaltite films. Adv Funct Mater 26:1564–1570
Karvonen L, Räsänen S, Yamauchi H, Karppinen M (2007) Chemical oxidation of SrCoO3−δ. Chem Lett 36:1176–1177
Karvonen L, Yamauchi H, Karppinen M (2008) Homologous series of SrCoO(3n−1)/n perovskites obtained through Br2 oxygenation of SrCoO2.5. Chem Mater 20:7143–7147
Ichikawa N, Iwanowska M, Kawai M, Calers C, Paulus W, Shimakawa Y (2012) Reduction and oxidation of SrCoO2.5 thin films at low temperatures. Dalton Trans 41:10507–10510
Nemudry A, Rudolf P, Schöllhorn R (1996) Topotactic electrochemical redox reactions of the defect perovskite SrCoO2.5+x. Chem Mater 8:2232–2238
Bezdicka P, Wattiaux A, Grenier J, Pouchard M, Hagenmuller P (1993) Preparation and characterization of fully stoichiometric SrCoO3 by electrochemical oxidation. Z Anorg Allg chem 619:7–12
Tambunan OT, Parwanta KJ, Acharya SK, Lee BW, Jung CU, Kim YS, Park BH, Jeong H, Park J-Y, Cho MR (2014) Resistance switching in epitaxial SrCoOx thin films. Appl Phys Lett 105:063507
Lu N, Zhang P, Zhang Q, Qiao R, He Q, Li H-B, Wang Y, Guo J, Zhang D, Duan Z, Li Z, Wang M, Yang S, Yan M, Arenholz E, Zhou S, Yang W, Gu L, Nan C, Wu J, Tokura Y, Yu P (2017) Electric-field control of tri-state phase transformation with a selective dual-ion switch. Nature 546:124–128
Lee JH, Rabe KM (2011) Coupled magnetic-ferroelectric metal-insulator transition in epitaxially strained SrCoO3 from first principles. Phys Rev Lett 107:067601
Lu Q, Yildiz B (2016) Voltage-controlled topotactic phase transition in thin-film SrCoOx monitored by in situ X-ray diffraction. Nano Lett 16:1186–1193
Hodges J, Jorgensen J, Miller D, Ma B, Balachandran U, Richardson J (1997) Crystal structures of mixed-conducting oxides present in the Sr–Fe–Co–O system. MRS Online Proc Libr Arch 496:173–178
Jeen H, Bi Z, Choi WS, Chisholm MF, Bridges CA, Paranthaman MP, Lee HN (2013) Orienting oxygen vacancies for fast catalytic reaction. Adv Mater 25:6459–6463
Tyler MJ, Rong X, Abakumov AM, Hardin WG, Dai S, Kolpak AM, Johnston KP, Stevenson KJ (2016) Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat Commun 7:11053
Hammouda SB, Zhao F, Safaei Z, Srivastava V, Ramasamy DL, Iftekhar S, Sillanpää M (2017) Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO3 (A = La, Ba, Sr and Ce): characterization, kinetics and mechanism study. Appl Catal B 215:60–73
Chen H, Yang M, Tao S, Ren M, Chen G (2016) Facile synthesis of Co3O4 with different morphologies via oxidation kinetic control and its application in hydrogen peroxide decomposition. Cryst Growth Des 16:6286–6293
Makhlouf MT, Abu-Zied B, Mansoure T (2013) Effect of calcination temperature on the H2O2 decomposition activity of nano-crystalline Co3O4 prepared by combustion method. Appl Surf Sci 274:45–52
Brodrecht DJ, Rusek JJ (2003) Aluminum–hydrogen peroxide fuel-cell studies. Appl Energy 74:113–124
Penna TCV, Ferraz CAM (2000) Cleaning of blood-contaminated reprocessed angiographic catheters and spinal needles. Infect Control Hosp Epidemiol 21:499–504
Dishman MV, Covey DA, Baughan LW (1994) The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater 10:33–36
Kuznetsova NI, Kuznetsova LI, Likholobov VA (1996) Catalytic properties of Cr-containing heteropolytungstates in H2O2 participated reactions: H2O2 decomposition and oxidation of unsaturated hydrocarbons with H2O2. J Mol Catal A Chem 108:135–143
Zaki MI, Katrib A, Muftah AI, Jagadale TC, Ikram M, Ogale SB (2013) Exploring anatase-TiO2 doped dilutely with transition metal ions as nano-catalyst for H2O2 decomposition: spectroscopic and kinetic studies. Appl Catal A 452:214–221
Huang X, Hou X, Zhao J, Zhang L (2016) Hematite facet confined ferrous ions as high efficient Fenton catalysts to degrade organic contaminants by lowering H2O2 decomposition energetic span. Appl Catal B 181:127–137
Volpe M, Ferreira ML, Eberhardt AM, Pedroni V (2004) Immobilization of catalase from Aspergillus niger on inorganic and biopolymeric supports for H2O2 decomposition. Appl Catal B 47:153–163
Pham AL, Doyle FM, Sedlak DL (2012) Inhibitory effect of dissolved silica on H2O2 decomposition by iron(III) and manganese(IV) oxides: implications for H2O2-based in situ chemical oxidation. Environ Sci Technol 46:1055
Vago ER, Calvo EJ (1995) Oxygen electro-reduction on iron oxide electrodes: III. Heterogeneous catalytic H2O2 decomposition. J Electroanal Chem 388:161–165
Chen F, Zhao X, Liu H, Qu J (2014) Reaction of Cu(CN) 2−3 with H2O2 in water under alkaline conditions: cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition. Appl Catal B 158–159:85–90
Zeineb O, Hedi BA, Jeday MR, Cheker C (2015) Kinetic study of the catalytic decomposition of H2O2 in phosphoric acid medium. Int J Hydrogen Energy 40:1278–1282
Shukla P, Sun H, Wang S, Ang HM, Tadé MO (2011) Nanosized Co3O4/SiO2 for heterogeneous oxidation of phenolic contaminants in waste water. Sep Purif Technol 77:230–236
Salem MA, Salem IA, Gemeay AH (1994) Kinetics and mechanism of H2O2 decomposition by Cu(II)-, Co(II)-, and Fe(III)-Amine complexes on the surface of Silica-Alumina (25% Al2O3). Int J Chem Kinet 26:1055–1061
Giordani V, Freunberger S, Bruce P, Tarascon J-M, Larcher D (2010) H2O2 decomposition reaction as selecting tool for catalysts in Li–O2 cells. Electrochem Solid State Lett 13:A180–A183
Micoli L, Bagnasco G, Turco M, Trifuoggi M, Sorge AR, Fanelli E, Pernice P, Aronne A (2013) Vapour phase H2O2 decomposition on Mn based monolithic catalysts synthesized by innovative procedures. Appl Catal B 140:516–522
Zhang W, Wang H, Yang Z, Wang F (2007) Promotion of H2O2 decomposition activity over β-MnO2 nanorod catalysts. Colloids Surf A 304:60–66
Lousada CM, Jonsson M (2010) Kinetics, mechanism, and activation energy of H2O2 decomposition on the surface of ZrO2. J Phys Chem C 114:11202–11208
Liu LD, Wang WM, Liu L, Yu B, Zhang YX, Wu XQ, Zhang HW, Han X (2016) Catalytic activities of dissolved and Sch-immobilized Mo in H2O2 decomposition: implications for phenol oxidation under acidic conditions. Appl Catal B 185:371–377
Zhang Q, Guan J, Rong R, Zhao Y, Yu Z (2015) Study on degradation kinetics of 2-(2-hydroxypropanamido) benzoic acid in aqueous solutions and identification of its major degradation product by UHPLC/TOF–MS/MS. J Pharm Biomed Anal 112:1–7
Petrie JR, Jeen HJ, Barron SC, Meyer TL, Lee HN (2016) J Am Chem Soc 138:7252–7255
Bokare AD, Choi W (2014) Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater 275:121–135
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61671206), Shanghai Science and Technology Innovation Action Plan (No. 17JC1402500) and Natural Science Foundation of Shanghai (No. 18ZR1410900).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 71807 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Cao, B., Lin, H. et al. H2O2 decomposition catalyzed by strontium cobaltites and their application in Rhodamine B degradation in aqueous medium. J Mater Sci 54, 8216–8225 (2019). https://doi.org/10.1007/s10853-019-03490-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03490-z