Abstract
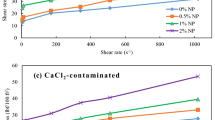
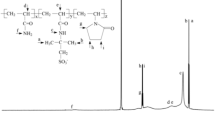
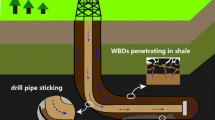
Bentonite/water-based drilling fluids (BT-WDFs) suffer serious fluid loss particularly at high temperatures, pressure levels, and divalent cation concentrations. The ionic liquid 1-vinyl-3-ethylimidazolium bromide was introduced to synthesize a filtration control additive (PASV) through free radical copolymerization with acrylamide and 2-acrylamido-2-methylpropanesulfonic acid. The effect of the concentration of the ionic liquid on the filtration property of the fluids was determined at high Ca2+ concentrations and temperatures. BT-WDFs added with 3.0 wt% copolymer exhibited excellent performance for controlling bivalent Ca2+-resistant fluid loss and tolerated the influence of Ca2+ up to 70 wt%, which approaches the saturation at room temperature (25 °C). As the PASV concentration increased to more than 1.0 wt%, the fluid loss volume was less than 10 mL at room temperature, thereby ensuring safe drilling operation. When the formations drilled were at high temperatures, the filtration control performance was obtained by adding PASV additives. The requisite concentration of PASV varied from 1.5 to 3.0% at 80–150 °C. An extremely dense filter cake was produced because of the elimination of the influence of Ca2+ on bentonite and the stabilization of the bentonite dispersion state by the PASV copolymer. These effects improved the filtration control performance of BT-WDFs. Moreover, the fluids exhibited shear thinning behavior over the shear rate range of 0.1–1000 s−1, resulting in improved practical drilling process. This study provides a basis for applying ionic liquids in the design of additives for drilling fluids.













Similar content being viewed by others
References
Vipulanandan C, Mohammed A (2015) Effect of nanoclay on the electrical resistivity and rheological properties of smart and sensing bentonite drilling muds. J Pet Sci Eng 130:86–95
Li M-C, Wu Q, Song K, Qing Y, Wu Y (2015) Cellulose nanoparticles as modifiers for rheology and fluid loss in bentonite water-based fluids. ACS Appl Mater Interfaces 7:5006–5016
Ghalambor A, Economides MJ (2000) Formation damage abatement: a quarter-century perspective. Paper presented at the SPE international symposium on formation damage control, Lafayette, Louisiana
Shakib JT, Kanani V, Pourafshary P (2016) Nano-clays as additives for controlling filtration properties of water–bentonite suspensions. J Pet Sci Eng 138:257–264
Barry MM, Jung Y, Lee J-K, Phuoc TX, Chyu MK (2015) Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J Pet Sci Eng 127:338–346
Kelessidis VC, Tsamantaki C, Michalakis A, Christidis GE, Makri P, Papanicolaou K, Foscolos A (2007) Greek lignites as additives for controlling filtration properties of water–bentonite suspensions at high temperatures. Fuel 86:1112–1121
Desbrieres J (1993) Cement cake properties in static filtration. Influence of polymeric additives on cement filter cake permeability. Cem Concr Res 23:347–358
Fattah KA, Lashin A (2016) Investigation of mud density and weighting materials effect on drilling fluid filter cake properties and formation damage. J Afr Earth Sci 117:345–357
Kosynkin DV, Ceriotti G, Wilson KC, Lomeda JR, Scorsone JT, Patel AD, Friedheim JE, Tour JM (2012) Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl Mater Interfaces 4:222–227
Duman O, Tunç S (2009) Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous Mesoporous Mater 117:331–338
Yoon J, El Mohtar CS (2015) Constitutive model parameters of concentrated bentonite suspensions modified with sodium pyrophosphate. J Mater Sci 50:5253–5261. doi:10.1007/s10853-015-9073-2
Choo KY, Bai K (2015) Effects of bentonite concentration and solution pH on the rheological properties and long-term stabilities of bentonite suspensions. Appl Clay Sci 108:182–190
Niriella D, Carnahan RP (2006) Comparison study of zeta potential values of bentonite in salt solutions. J Dispers Sci Technol 27:123–131
Cadix A, Wilson J, Barthet C, Phan C, Poix C, Dupuis P, Harrisson S (2015) Diblock copolymers: a new class of fluid loss control additive for oilfield cementing. Paper presented at the SPE international symposium on oilfield chemistry. The Woodlands, Texas, USA
Xue Z, Foster E, Wang YG, Nayak S, Cheng V, Ngost VW, Pennell KD, Bielawski CW, Johnston KP (2014) Effect of grafted copolymer composition on iron oxide nanoparticle stability and transport in porous media at high salinity. Energy Fuel 28:3655–3665
Sehlleier YH, Abdali A, Schnurre SM, Wiggers H, Schulz C (2014) Surface functionalization of microwave plasma-synthesized silica nanoparticles for enhancing the stability of dispersions. J Nanopart Res 16:1–11
Bagaria HG, Yoon KY, Neilson BM, Cheng V, Lee JH, Worthen AJ, Xue Z, Huh C, Bryant SL, Bielawski CW, Johnston KP (2013) Stabilization of iron oxide nanoparticles in high sodium and calcium brine at high temperatures with adsorbed sulfonated copolymers. Langmuir 29:3195–3206
Plank J, Brandl A, Lurnmer NR (2007) Effect of different anchor groups on adsorption behavior and effectiveness of poly (N,N-dimethylacrylamide-co-Ca 2-acrylamido-2.7 methylpropanesulfonate) as cement fluid loss additive in presence of acetone-formaldehyde-sulfite dispersant. J Appl Polym Sci 106:3889–3894
Khoshniyat A, Hashemi A, Sharif A, Aalaie J, Duobis C (2012) Effect of surface modification of bentonite nanoclay with polymers on its stability in an electrolyte solution. Polym Sci Ser B 54:61–72
Luckham PF, Rossi S (1999) The colloidal and rheological properties of bentonite suspensions. Adv Colloid Interface Sci 82:43–92
Guancheng Jiang FL, Ren Yanjun, Wang Lan, Luo Taotao, Deng Zhengqiang, Cheng Zepu (2015) Synthesis and evaluation of high temperature resistant and high calcium tolerant filtrate loss additive DF-1. Oilfield Chem 32:6
Liu F, Jiang GC, Peng SL, He YB, Wang JX (2016) Amphoteric polymer as an anti-calcium contamination fluid-loss additive in water-based drilling fluids. Energy Fuel 30:7221–7228
Wong KY, Jen AKY (1994) Thermally stable poled polyimides using heteroaromatic chromophores. J Appl Phys 75:3308–3310
Endo K, Tatsumi T (1997) Amorphous carbon thin films containing benzene rings for use as low-dielectric-constant interlayer dielectrics. Appl Phys Lett 70:2616–2618
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev 111:3508–3576
Gou SH, Yin T, **a Q, Guo QP (2015) Biodegradable polyethylene glycol-based ionic liquids for effective inhibition of shale hydration. RSC Adv 5:32064–32071
Gou S, Yin T, Yan L, Guo Q (2015) Water-soluble complexes of hydrophobically modified polymer and surface active imidazolium-based ionic liquids for enhancing oil recovery. Colloids Surf A Physicochem Eng Asp 471:45–53
Vicent-Luna JM, Dubbeldam D, Gomez-Alvarez P, Calero S (2016) Aqueous solutions of ionic liquids: microscopic assembly. ChemPhysChem 17:380–386
Green MD, Long TE (2009) Designing imidazole-based ionic liquids and ionic liquid monomers for emerging technologies. Polym Rev 49:291–314
RP2A-WSD A (2007) American petroleum institute recommended practice for planning, designing and constructing fixed offshore platforms—working stress design. American Petroleum Institute, Washington
Jönsson B, Labbez C, Cabane B (2008) Interaction of nanometric clay platelets. Langmuir 24:11406–11413
Segad M, Hanski S, Olsson U, Ruokolainen J, Akesson T, Jonsson B (2012) Microstructural and swelling properties of Ca and Na montmorillonite: (in situ) observations with Cryo-TEM and SAXS. J Phys Chem C 116:7596–7601
Norrish K (1954) Crystalline swelling of montmorillonite: manner of swelling of montmorillonite. Nature 173:256–257
Santillan EFU, Choi W, Bennett PC, Leyris JD (2015) The effects of biocide use on the microbiology and geochemistry of produced water in the Eagle Ford formation, Texas, USA. J Pet Sci Eng 135:1–9
Plank J, Brandl A, Zhai YN, Franke A (2006) Adsorption behavior and effectiveness of poly(N,N-dimethylacrylamide-co-Ca 2-acrylamido-2-methylpropanesulfonate) as cement fluid loss additive in the presence of acetone-formaldehyde-sulfite dispersant. J Appl Polym Sci 102:4341–4347
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 51604290) and startup foundation of China University of Petroleum (Bei**g) (Grant No. 2462015YJRC023).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10853-017-0920-1.
Rights and permissions
About this article
Cite this article
Yang, L., Jiang, G., Shi, Y. et al. Application of ionic liquid to a high-performance calcium-resistant additive for filtration control of bentonite/water-based drilling fluids. J Mater Sci 52, 6362–6375 (2017). https://doi.org/10.1007/s10853-017-0870-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0870-7