Abstract
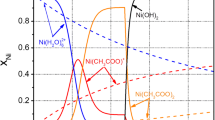
This article focuses on the nanoparticle electrocatalytical action on electrodeposition of nickel and the chemical combination state between nanoparticles and matrix metal in composite coating. The electrochemical behavior, from common and composite brush electroplating solution, is investigated by cyclic voltammetry. The interaction between nanoparticles and matrix metal nickel is researched by X-Ray Photoelectron Spectrometry (XPS). The microstructure and morphology of coating are observed with Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM). The results show that nanoparticles not only can obviously induce the increasing of the current efficiency and decreasing of overpotential, but also can distinctly refine the metal crystal grains of composite coating. The experimental results demonstrate that nanoparticles take part in the electrode reaction and can evidently catalyze nickel electrodeposition. Part of the unsaturated oxygen atoms on nanoparticles surface can combine with some of the absorbed nickel atoms and form nickel-oxygen chemical bonds. There is chemical binding interaction at the interface between nanoparticles’ surface and matrix metal nickel.










Similar content being viewed by others
References
Vanek D (2002) Met Finish 7:18
Dini JW (1997) Met Finish 6:88
Subramanian B, Sanjeeviraja C, Jayachandran M (2002) J Crystal Growth 234:421
Hu SB, Tu JP, Mei Z (2001) Surf Coat Tech 141:174
Clarke RD (1999) Int J Adhes Adhes 19:205
Yang RP, Cai X, Chen QL (2001) Surf Coat Techn 141:283
Ma YJ, Zhu ZX (2001) Surf Techn 30(6):5
Tu WY, Xu BS, Jiang B, Dong SY (2003) Chinese J Mater Res 10(5):530
Wang W, Gau HT, Gao JP, Qin QX, Ren DS (1997) Chinese J Mater Res 11(2):143
Netravall AN (1989) Compos Sci Technol 34:289
Xu B-S, Dong S-Y, Liang Z-J, Wang H-M, Hu Z-F, A preparation method on nanoparticles composite electro-brush plating solution [Patent], China, 02101196.6, 2002.08
Xu BS, Wang HD, Dong SY, Jiang B, Tu WY (2004) T Nonferr Metal Soc 14(special 2):111
Xue QJ, Xu K (2000) Prog Chem 12(4):431
Galtayries A, Grimblot J (1999) J Electron Spectrosc 98/99:267
Wanger CD, Riggs WM, Davis LE et al. (1979) Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corporation Physical Electronic Division, Minnesota
Abyaneh MY, Fleischmann M (1981) J Electroanal Chem 119:187
Abyaneh MY, Fleischmann M (1981) J Electroanal Chem 119:197
Amblard J, Forment M, Maurin G, Spyrellis N, Trevisan-Southyrand E (1983) Elecrtochemica Acta 28(7):909
Weiyi T, Binshi X, Shiyun D, Bin J, Lingzhong D, Zhenfeng H (2005) T Nonferr Metals Soc 15(4):889
Yin MZ, Yao X, Wu XQ (2003) Chinese J Mater Res 17(2):220
Jiang B, Xu B, Dong S, Ding P (2005) Heat Treat Met 30(6):5
Acknowledgements
The work was supported by National Natural Science Foundation of China (No. 50235030), 973 Plan of National (No. G1999065009), and Cooperative Foundation of Science and Technology of Sino-Britain Government (No. 2002M3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, WY., Xu, BS., Dong, SY. et al. Chemical and electrocatalytical interaction: influence of non-electroactive ceramic nanoparticles on nickel electrodeposition and composite coating. J Mater Sci 43, 1102–1108 (2008). https://doi.org/10.1007/s10853-007-2259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2259-5